Cory:
Unlock Your AI Assistant Now!
Abstract
Background: New conduction disturbances are frequent after transcatheter aortic valve implantation (TAVI). Refining our ability to predict high-grade atrioventricular block (AVB) occurring later than 24 hours after the procedure would be useful in order to select patients eligible for early discharge.
Aims: This study was designed to identify predictors of high-grade AVB occurring between 24 hours and 30 days after TAVI and to develop and validate a predictive risk score.
Methods: We analysed clinical, procedural, and electrocardiographic parameters of 1,290 TAVI patients. Independent predictors of delayed high-grade AVB were used to develop the predictive score, which was then externally validated in a cohort of 936 patients.
Results: Implantation of self-expanding valves, greater implantation depth, longer PR interval in preprocedural electrocardiogram (ECG) and greater increase of PR duration in next-day ECG, preprocedural right bundle branch block (RBBB) and new-onset left bundle branch block or RBBB that persisted in next-day ECG were independent predictors of delayed high-grade AVB and were combined to develop the Delayed atrioventricular block Prediction for eArly disChargE (D-PACE) score. The areas under the curve of the score were 0.879 (95% confidence interval [CI]: 0.835-0.923) and 0.799 (95% CI: 0.730-0.868) in the derivation and validation cohorts, respectively. Based on the score, patients can be classified into three risk categories; low-risk patients demonstrated an incidence of delayed AVB of less than 1% and are ideal candidates for next-day discharge.
Conclusions: The D-PACE score can be used to stratify TAVI patients according to their risk of delayed high-grade AVB and thereby identify those suitable for next-day discharge.
Despite significant improvements, the occurrence of high-grade atrioventricular block (AVB), with subsequent permanent pacemaker implantation (PPI), remains a relatively common event after transcatheter aortic valve implantation (TAVI) and is one of the most frequent complications1. Most high-grade AVBs (60% to 96%) occur in the first 24 hours following the procedure234. AVBs occurring after this time frame are generally considered delayed, although there is no universally accepted definition.
Even though TAVI allows patient mobilisation as early as a few hours after the procedure in most cases56, the risk of delayed AVB frequently results in prolonged hospitalisation for continuous electrocardiogram (ECG) monitoring7. A recent European survey8 showed that only 12% of patients are discharged the day after the procedure and only 31% on the second day.
Although still not widely adopted, next-day discharge has proved to be a safe strategy: in large retrospective studies910, patients who were discharged the day after the procedure had similar outcomes, including late PPI, compared to those who had a longer hospital stay. A significant limitation of these studies is that, because of their retrospective design, they include patients with a very low risk of delayed conduction disturbances.
To promote early discharge, Rodés-Cabau et al proposed an algorithm for postprocedural patient management, based on electrocardiographic parameters11. In a subsequent validation study of this algorithm, 45% of patients were identified as potential candidates for next-day discharge, but nearly 4% of them required PPI at 30 days12.
Against this background, the main aim of the present study was to identify predictors of high-grade AVB occurring between 24 hours and 30 days after TAVI and to develop a readily applicable risk-stratification tool allowing the identification of low-risk patients who are suitable for hospital discharge 24 hours after the procedure.
Methods
Study population
We evaluated consecutive patients treated with TAVI at the University Hospital of Bologna between March 2014 and June 2023 (group A) to identify the predictors of delayed high-grade AVB requiring PPI and to develop a predictive model. A second, combined retrospective and prospective cohort (group B), consisting of patients treated at the University Hospital of Bologna between July 2023 and March 2024 and at the University Hospital of Catania between January 2018 and March 2024, was used to validate the model. Patients with a permanent pacemaker or implantable cardioverter-defibrillator with pacing function before TAVI were excluded. We also excluded patients who developed high-grade AVB in the first 24 hours after the procedure, those who underwent PPI in the first 30 days after TAVI for reasons other than high-grade AVB, those who died in the first 30 days and had not developed high-grade AVB, and those for whom 30-day follow-up was not available in the clinical records. Finally, only patients with all the following periprocedural ECGs available were included:
⢠Preprocedural: last ECG before TAVI recorded no more than 72 hours before the procedure.
⢠Postprocedural: last ECG recorded within 4 hours after TAVI.
⢠Next day: last ECG recorded between 12 hours and 24 hours after TAVI.
This study conforms to the principles of the Declaration of Helsinki. The local ethics committees approved the protocol.
Data acquisition and endpoint
The primary endpoint of the study was high-grade AVB occurring between 24 hours and 30 days after TAVI. Third-degree, second-degree type 2, 2:1 or advanced (3:1, 4:1, etc.) AVBs were included in this category. These atrioventricular conduction disturbances represent a clear indication for PPI, regardless of persistent or paroxysmal nature or presence of associated symptoms (Class I recommendation in the European Society of Cardiology guidelines on cardiac pacing from 202113).
We evaluated medical history, symptoms, echocardiographic and laboratory data, as well as procedural characteristics for all patients. Patients who underwent a valve-in-valve procedure were classified into two groups: one including patients treated for dysfunction of conventional surgical valves and a second including patients who had previously undergone implantation of transcatheter or sutureless surgical valves. The latter two types of valves were combined because they share a similar structure that sets them apart from traditional surgical valves. Unlike conventional valves, they lack a rigid ring, and a portion of the valve is located below the annular plane, within the left ventricular outflow tract (LVOT).
ECGs and implantation depth were analysed by investigators blinded to the endpoint occurrence. Atrioventricular and intraventricular conduction disturbances were defined according to the American Heart Association, American College of Cardiology, and Heart Rhythm Society consensus document on ECG interpretation14 and guidelines on the evaluation and management of patients with bradycardia and cardiac conduction delays15. Intraventricular conduction disturbances that occurred after the procedure were considered transient if they were present in the postprocedural ECG but had resolved in the next-day ECG, whereas they were considered persistent if they were still present. Implantation depth was measured from angiographic images of the TAVI procedure, following the method used in other studies1617, as the distance between the lowest point of the non-coronary cusp and the lower edge of the valve stent in a projection where the three cusps are aligned on the same plane (cusp-overlap or 3-cusp projections) (Supplementary Figure 1).
Statistical analysis
Continuous variables are reported as mean values and standard deviation or as median and interquartile range (IQR; 25th-75th) in case of normal or non-normal distribution (the normality of the distribution was verified with the Shapiro-Wilk test). The Student’s t-test or the Mann-Whitney U test were used to compare groups, as appropriate. Categorical variables are reported as percentages and were compared using the chi-square test or Fisher’s exact test. The relationship between individual variables and the study endpoint was assessed by univariate logistic regression. Variables with a p-value<0.10 in univariate analysis were included in the multivariate logistic regression model with stepwise backward elimination. Interactions between independent predictors of delayed high-grade AVB were tested. The discriminative capacity of the multivariate model was assessed using the area under the receiver operating characteristic (ROC) curve. Goodness of fit was tested with the Hosmer-Lemeshow test, withp>0.05 corresponding to a good fit.
Clinical risk score development and validation
A point risk score was developed based on the results of multivariate logistic regression, using the method described by Sullivan et al18. Each independent variable was assigned a weight proportional to its regression coefficient. Continuous variables were divided into categories of increasing risk. The patient’s score was obtained from the sum of the points given for the individual independent predictors. Subsequently, the score was subjected to univariate logistic regression analysis in relation to the endpoint among patients in the validation cohort. The discrimination of the score was assessed using the area under the ROC curve, and its calibration was evaluated with the Hosmer-Lemeshow test in both the derivation and validation cohorts. A 2-tailed alpha of 0.05 was used to define the significance threshold for all comparisons.
Statistical analyses were performed using Stata 17 (StataCorp) and R, version 4.0.4 (R Foundation for Statistical Computing).
Results
Study population and outcomes
Of the 1,833 patients who underwent TAVI at the University Hospital of Bologna between March 2014 and June 2023, 1,290 met the inclusion/exclusion criteria (Supplementary Figure 2) and were included in the study (group A). Seventy-four patients (5.7%) developed high-grade AVB between 24 hours and 30 days after TAVI. The median time between the TAVI procedure and high-grade AVB onset was 3 days (IQR 2-5 days). All patients who developed delayed high-grade AVB subsequently underwent successful PPI, and none of them presented with sudden death/need of resuscitation.
Baseline clinical and echocardiographic characteristics of patients in group A are shown in Table 1. Patients who developed high-grade AVB did not differ significantly from the remaining population in terms of demographic characteristics, cardiovascular risk factors, and comorbidities. None of the 92 patients who underwent TAVI for dysfunction of a conventional surgical valve developed high-grade AVB, while this event occurred in 2 of the 6 patients with transcatheter or sutureless surgical valves (0.0% vs 7.6%, and 2.7% vs 0.3%, respectively; p<0.001).
Procedural and ECG characteristics are shown in Table 2. Implantation of self-expanding valves was more frequent in patients who developed AVB (40.5% vs 25.6%; p=0.005; additional data regarding specific valve models are reported (Supplementary Appendix 1, Supplementary Table 1, Supplementary Table 2). In addition, implantation depth was greater (6.0 [IQR 5.0-7.2] mm vs 4.3 [IQR 3.4-5.3]mm; p<0.001) and transient procedural high-grade AVB episodes were more frequent (8.1% vs 2.5%; p=0.014) in this group. Regarding ECG variables, the AVB population showed a longer duration of preprocedural PR interval (200 [IQR 173-231] ms vs 176 [IQR 160-202] ms; p<0.001) and a greater increase in its duration in next-day ECG (+10 [IQR –2 to +48] ms vs –2 [IQR –12 to +10] ms; p<0.001), but not in postprocedural ECG (+9 [IQR –6 to +22] ms vs +4 [IQR –6 to +14] ms; p=0.129). Preprocedural right bundle branch block (RBBB) was more frequent in those who developed AVB (28.4% vs 9.9%; p<0.001), while left bundle branch block (LBBB) was not (5.4% vs 9.5%; p=0.304). New occurrences of both LBBB and RBBB that persisted in next-day ECG were more frequent in the AVB group (28.4% vs 9.1%; p<0.001, and 4.0% vs 0.6%; p=0.016, respectively), while there was no difference for transient bundle branch blocks.
Table 1. Clinical history and echocardiographic features of patients from group A.
| Variables | Delayed AVB (n=74) | No AVB (n=1,216) | p-value |
|---|---|---|---|
| Age, years | 84 (82-87) | 84 (81-87) | 0.733 |
| Male sex | 51.4 | 45.1 | 0.292 |
| BMI, kg/m2 | 27.0 (24.2-29.1) | 25.6 (23.1-28.7) | 0.073 |
| Hypertension | 92.9 | 90.9 | 0.767 |
| Dyslipidaemia | 60.8 | 69.4 | 0.121 |
| Diabetes | 25.7 | 26.0 | 0.953 |
| Smoker | 33.8 | 38.7 | 0.395 |
| NYHA Class III-IV | 44.6 | 53.8 | 0.124 |
| Prior syncope | 12.2 | 11.7 | 0.900 |
| Prior myocardial infarction | 23.0 | 19.4 | 0.453 |
| Coronary artery disease | 46.0 | 52.5 | 0.276 |
| Prior balloon aortic valvuloplasty | 40.5 | 46.1 | 0.355 |
| Atrial fibrillation/flutter | 43.2 | 33.1 | 0.074 |
| Prior stroke | 5.4 | 7.7 | 0.650 |
| Peripheral artery disease | 24.3 | 26.2 | 0.717 |
| Native valve disease vs bioprosthesis dysfunction | <0.001 | ||
| Native aortic valve disease | 97.3 | 92.1 | |
| Prior SAVR with conventional bioprosthesis | 0.0 | 7.6 | |
| Prior TAVI or SAVR with sutureless bioprosthesis | 2.7 | 0.3 | |
| Prior non-SAVR cardiac surgery | 12.2 | 7.2 | 0.111 |
| Bicuspid aortic valve | 1.4 | 3.3 | 0.727 |
| STS score, % | 4.8 (3.4-6.7) | 4.3 (3.0-6.4) | 0.202 |
| EuroSCORE II, % | 4.1 (2.9-6.9) | 4.2 (2.6-6.8) | 0.712 |
| Estimated glomerular filtration rate, ml/min/1.73 m2 | 49 (37-67) | 55 (41-70) | 0.059 |
| Left ventricular end-diastolic volume, ml | 90 (78-114) | 93 (74-118) | 0.957 |
| Left ventricular ejection fraction <50% | 13.5 | 19.5 | 0.205 |
| Interventricular septum thickness, mm | 13 (13-14) | 13 (12-14) | 0.031 |
| Aortic valve area (indexed to BSA), cm2/m2 | 0.39 (0.35-0.47) | 0.41 (0.35-0.47) | 0.839 |
| Values are expressed as median (interquartile range) or as %. AVB: atrioventricular block; BMI: body mass index; BSA: body surface area; EuroSCORE: European System for Cardiac Operative Risk Evaluation; NYHA: New York Heart Association; SAVR: surgical aortic valve replacement; STS: Society of Thoracic Surgeons; TAVI: transcatheter aortic valve implantation | |||
Table 2. Procedural and electrocardiographic features of patients from group A.
| Variables | Delayed AVB (n=74) | No AVB (n=1,216) | p-value |
|---|---|---|---|
| Self-expanding valve | 40.5 | 25.6 | 0.005 |
| Non-transfemoral access | 4.1 | 4.8 | 1.000 |
| Valve post-dilatation | 8.1 | 6.8 | 0.852 |
| Implantation depth, mm | 6.0 (5.0-7.2) | 4.3 (3.4-5.3) | <0.001 |
| Transient procedural AVB | 8.1 | 2.5 | 0.014 |
| Preprocedural HR, bpm | 69 (60-78) | 68 (60-77) | 0.620 |
| Preprocedural PR interval, ms (n=1,005) | 200 (173-231) | 176 (160-202) | <0.001 |
| Preprocedural LBBB | 5.4 | 9.5 | 0.304 |
| Preprocedural RBBB | 28.4 | 9.9 | <0.001 |
| Preprocedural LAFB | 21.6 | 13.6 | 0.056 |
| Postprocedural HR variation*, bpm | +1 (–9 to +9) | +2 (–5 to +10) | 0.192 |
| Postprocedural PR variation*, ms (n=991) | +9 (–6 to +22) | +4 (–6 to +14) | 0.129 |
| Next-day HR variation^, bpm | +4 (–1 to +12) | +8 (0 to +16) | 0.068 |
| Next-day PR variation^, ms (n=987) | +10(–2 to +48) | –2(–12 to +10) | <0.001 |
| New-onset LBBB | |||
| Persistent† | 28.4 | 9.1 | <0.001 |
| Transient‡ | 1.4 | 4.3 | 0.363 |
| New-onset RBBB | |||
| Persistent† | 4.0 | 0.6 | 0.016 |
| Transient‡ | 1.4 | 0.3 | 0.256 |
| New-onset LAFB | |||
| Persistent† | 1.4 | 1.2 | 0.614 |
| Transient‡ | 1.4 | 0.6 | 0.377 |
| Values are expressed as median (interquartile range) or as %. Data available for 1,290 patients unless otherwise specified. *Difference between values on postprocedural and preprocedural ECGs. ^Difference between values on next-day and preprocedural ECGs. †Conduction disturbances are considered persistent if they are present on both postprocedural and next-day ECGs. ‡Conduction disturbances are considered transient if they are present on a postprocedural ECG but not on the next-day ECG. AVB: atrioventricular block; ECG: electrocardiogram; HR: heart rate; LAFB: left anterior fascicular block; LBBB: left bundle branch block; RBBB: right bundle branch block | |||
Logistic regression analysis and risk score development
Valve-in-valve patients with conventional surgical valves were not included in the regression model, as none of these patients developed the event. Logistic regression results are shown in Table 3. In multivariate analysis, independent predictors of delayed high-grade AVB were implantation of self-expanding valves, greater implantation depth, longer duration of PR interval in preprocedural ECG and a greater increase of the PR interval duration in next-day ECG, preprocedural RBBB, and new onset of LBBB or RBBB that persisted in next-day ECG. Because two of the independent predictors required measurement of the PR interval, the final logistic regression was performed only on patients in whom this interval was measurable in both preprocedural and next-day ECG (derivation cohort; n=915). The multivariate model showed good performance on ROC curve analysis (area under the curve [AUC] 0.889), and the Hosmer-Lemeshow χ2 was 755.90 (p=0.999).
Using the adjusted regression coefficients of the final multivariate regression model, we developed an integer risk score named Delayed atrioventricular block Prediction for eArly disChargE (D-PACE) (Central illustration). Possible values of the score range from 0 to 14, while the values in the derivation cohort ranged from 0 to 12 (Figure 1). The odds ratio (OR) for each increasing point of the score was 1.954 (95% confidence interval [CI]: 1.693-2.255; p<0.001). ROC curve analysis showed an AUC of 0.879 (95% CI: 0.835-0.923) (Figure 2), and the Hosmer-Lemeshow χ2 was 13.94 (p=0.236), indicating good discrimination and calibration.
Three risk categories were defined, according to predicted 30-day AVB risk (Supplementary Table 3):
⢠Low risk: D-PACE score from 0 to 3, corresponding to a predicted AVB risk <2%.
⢠Intermediate risk: D-PACE score from 4 to 5, corresponding to a predicted AVB risk between 2% and 5%.
⢠High risk: D-PACE score ≥6, corresponding to a predicted AVB risk ≥5%.
In the derivation cohort, 48.6% of the patients were classified as low risk, 30.4% as intermediate risk and 21.0% as high risk. Figure 3 displays the observed and predicted risks of delayed AVB across the three risk groups. Observed and predicted AVB incidence was similar, indicating good calibration of the model.
Table 3. Logistic regression model.
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR | p-value | OR | p-value | |
| Age | 1.014 (0.977-1.058) | 0.501 | ||
| Male sex | 1.287 (0.804-2.064) | 0.293 | ||
| BMI | 1.027 (0.978-1.074) | 0.269 | ||
| Hypertension | 1.138 (0.523-2.991) | 0.767 | ||
| Dyslipidaemia | 0.684 (0.424-1.118) | 0.123 | ||
| Diabetes | 0.984 (0.561-1.654) | 0.953 | ||
| Smoking | 0.807 (0.485-1.312) | 0.396 | ||
| NYHA Class III-IV | 0.692 (0.429-1.107) | 0.126 | ||
| Prior syncope | 1.047 (0.477-2.044) | 0.900 | ||
| Prior myocardial infarction | 1.238 (0.688-2.122) | 0.454 | ||
| Coronary artery disease | 0.770 (0.479-1.232) | 0.277 | ||
| Prior balloon aortic valvuloplasty | 0.799 (0.491-1.282) | 0.356 | ||
| Atrial fibrillation/flutter | 1.537 (0.950-2.466) | 0.076& | ||
| Prior stroke | 0.690 (0.207-1.713) | 0.480 | ||
| Peripheral artery disease | 0.904 (0.510-1.530) | 0.717 | ||
| Prior TAVI or SAVR with sutureless valve | 7.778 (1.066-40.540) | 0.019& | ||
| Prior non-SAVR cardiac surgery | 1.797 (0.812-3.556) | 0.116 | ||
| Bicuspid aortic valve | 0.403 (0.023-1.894) | 0.372 | ||
| STS score | 1.017 (0.961-1.062) | 0.502 | ||
| EuroSCORE II | 0.991 (0.940-1.031) | 0.708 | ||
| Estimated glomerular filtration rate | 0.988 (0.976-0.999) | 0.046& | ||
| Left ventricular end-diastolic volume | 0.998 (0.992-1.004) | 0.560 | ||
| Left ventricular ejection fraction <50% | 0.645 (0.327-1.276) | 0.208 | ||
| Interventricular septum thickness | 1.111 (0.973-1.260) | 0.110 | ||
| Aortic valve area (indexed to BSA) | 1.329 (0.179-7.535) | 0.765 | ||
| Self-expanding valve | 1.984 (1.216-3.198) | 0.005& | 2.166 (1.075-4.366) | 0.031& |
| Non-transfemoral access | 0.844 (0.202-2.356) | 0.778 | ||
| Valve post-dilatation | 1.204 (0.456-2.646) | 0.673 | ||
| Implantation depth per mm | 1.472 (1.325-1.638) | <0.001& | 1.463 (1.266-1.691) | <0.001& |
| Transient procedural AVB | 3.488 (1.275-8.124) | 0.007& | ||
| Preprocedural HR per bpm | 1.004 (0.987-1.020) | 0.608 | ||
| Preprocedural PR interval per ms | 1.014 (1.008-1.020) | <0.001& | 1.016 (1.009-1.023) | <0.001& |
| Preprocedural LBBB | 0.547 (0.164-1.351) | 0.249 | ||
| Preprocedural RBBB | 3.619 (2.072-6.125) | <0.001& | 5.569 (2.359-13.145) | <0.001& |
| Preprocedural LAFB | 1.745 (0.950-3.037) | 0.058& | ||
| Postprocedural HR variation* per bpm | 0.986 (0.968-1.004) | 0.120 | ||
| Postprocedural PR variation* per ms | 1.014 (1.002-1.025) | 0.017& | ||
| Next-day HR variation^ per bpm | 0.986 (0.970-1.003) | 0.104 | ||
| Next-day PR variation^ per ms | 1.029 (1.020-1.038) | <0.001& | 1.029 (1.019-1.040) | <0.001& |
| New-onset LBBB | ||||
| Persistent† | 3.944 (2.253-6.693) | <0.001& | 4.488 (2.011-10.014) | <0.001& |
| Transient‡ | 0.301 (0.169-1.399) | 0.237 | ||
| New-onset RBBB | ||||
| Persistent† | 7.298 (1.548-26.860) | 0.005& | 9.283 (1.119-77.037) | 0.039& |
| Transient‡ | 4.151 (0.211-28.450) | 0.206 | ||
| New-onset LAFB | ||||
| Persistent† | 1.097 (0.060-5.526) | 0.929 | ||
| Transient‡ | 2.366 (0.126-13.544) | 0.423 | ||
| Values are expressed as median (interquartile range). &Indicates statistical significance. *Difference between values on postprocedural and preprocedural ECGs. ^Difference between values on next-day and preprocedural ECGs. †Conduction disturbances are considered persistent if they are present on both postprocedural and next-day ECGs. ‡Conduction disturbances are considered transient if they are present on a postprocedural ECG but not on the next-day ECG. AVB: atrioventricular block; BMI: body mass index; BSA: body surface area; ECG: electrocardiogram; EuroSCORE: European System for Cardiac Operative Risk Evaluation; HR: heart rate; LAFB: left anterior fascicular block; LBBB: left bundle branch block; NYHA: New York Heart Association; OR: odds ratio; RBBB: right bundle branch block; SAVR: surgical aortic valve replacement; STS: Society of Thoracic Surgeons; TAVI: transcatheter aortic valve implantation | ||||
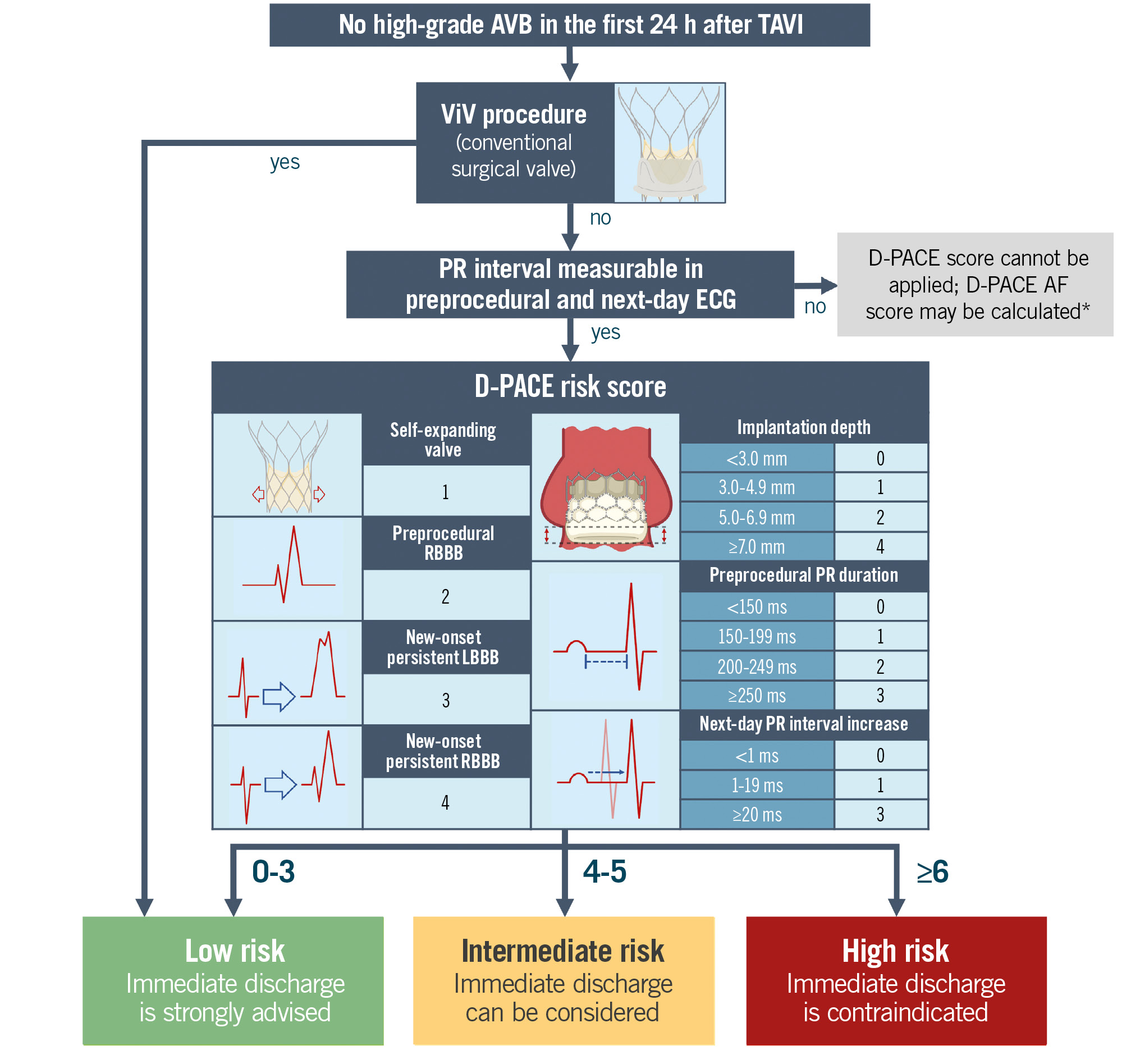
Central illustration. Algorithm for delayed high-grade AVB risk stratification. The algorithm should be applied 24 hours after TAVI if there have been no episodes of high-grade AVB in this time interval. Bundle branch blocks are considered persistent if they are present in both postprocedural and next-day (12-24 hours after TAVI) ECGs. Next-day PR variation is the difference in PR interval duration between preprocedural and next-day ECGs. *The D-PACE AF score requires additional validation, and there is no established cutoff for suggesting early discharge. Image adapted from Biorender.com. AF: atrial fibrillation/flutter; AVB: atrioventricular block; D-PACE: Delayed atrioventricular block Prediction for eArly disChargE; ECG: electrocardiogram; LBBB: left bundle branch block; RBBB: right bundle branch block; TAVI: transcatheter aortic valve implantation; ViV: valve-in-valve
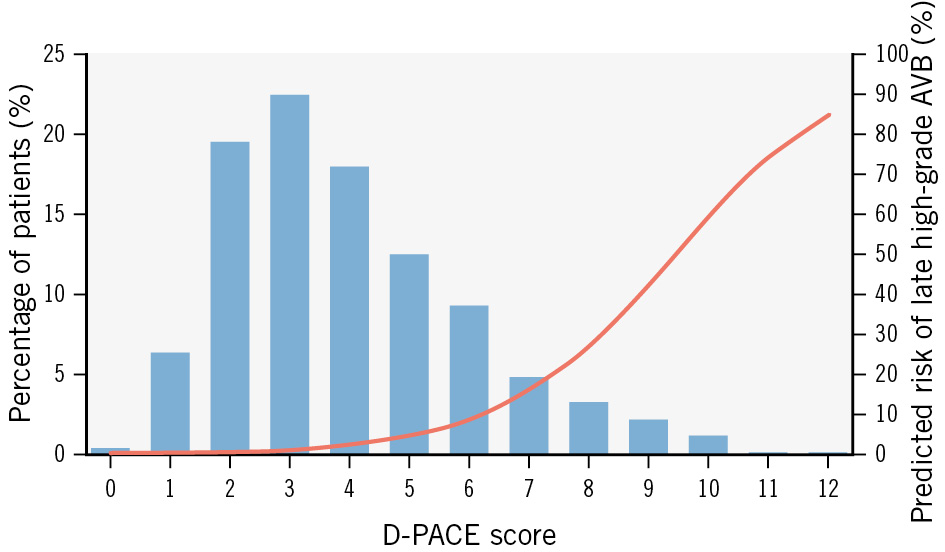
Figure 1. Distribution of D-PACE scores in the derivation cohort and predicted AVB incidence. Blue bars show the percentage of patients from the derivation cohort for each score value; the red line displays the mean predicted probability of late-onset high-grade AVB for each score value. AVB: atrioventricular block; D-PACE: Delayed atrioventricular block Prediction for eArly disChargE
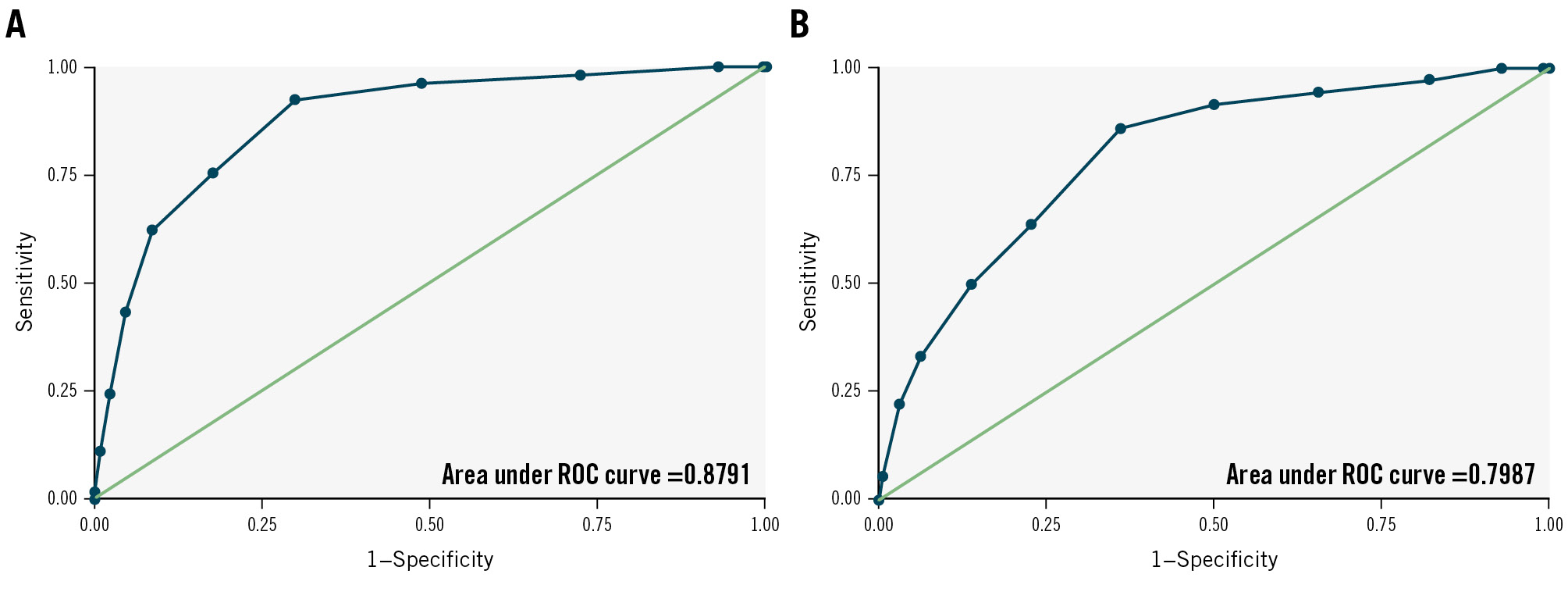
Figure 2. Receiver operating characteristic curves for the D-PACE score. A) Curve from the derivation cohort (area under the curve [AUC] 0.879, 95% confidence interval [CI]: 0.835-0.923). B) Curve from the validation cohort (AUC 0.799, 95% CI: 0.730-0.868). D-PACE: Delayed atrioventricular block Prediction for eArly disChargE; ROC: receiver operating characteristic
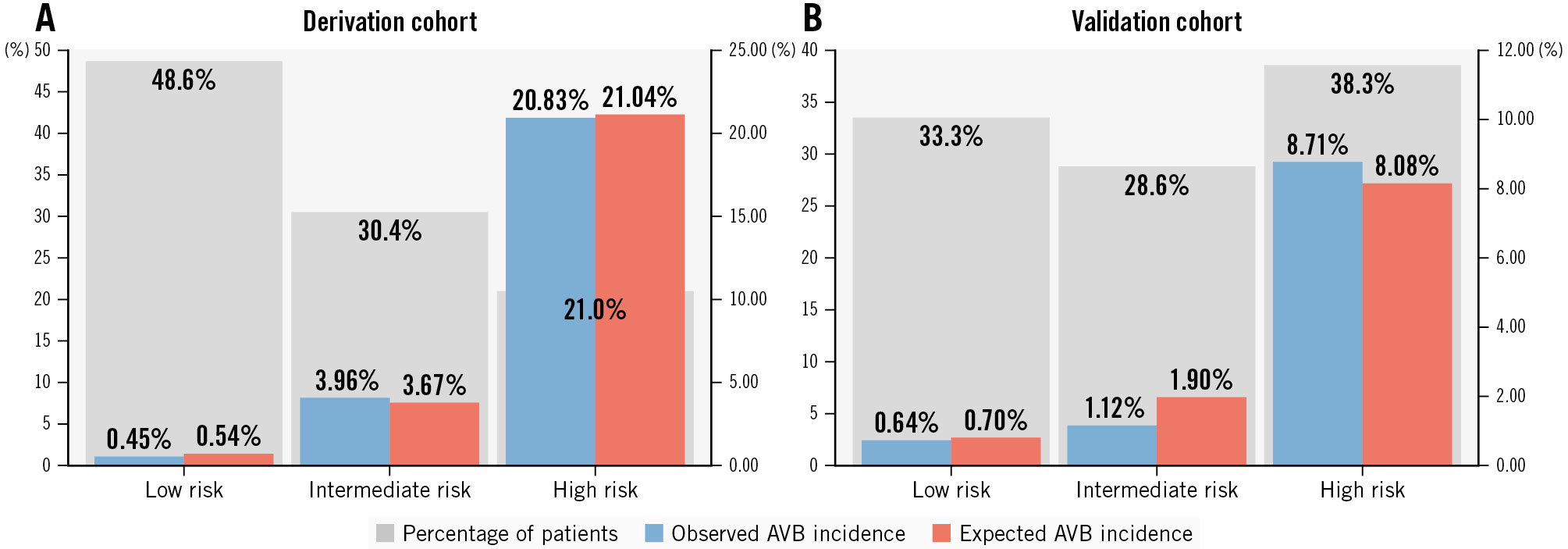
Figure 3. Distribution of patients across the three risk categories and observed and predicted rates of delayed high-grade AVB in each category. Grey bars show the percentage of patients in each risk category; blue bars show the observed event rate; red bars show the predicted event rate. Observed and predicted risks were similar in both the derivation (A) and validation (B) cohorts, indicating good calibration of the model. AVB: atrioventricular block
Risk score validation
Group B consisted of 1,226 patients who met the inclusion criteria (Supplementary Figure 2). Of these, 936 were included in the validation cohort, after excluding 33 patients who underwent valve-in-valve TAVI for conventional surgical valve dysfunction (none of them developed delayed AVB) and 257 patients without a measurable PR interval in preprocedural and/or next-day ECG. Thirty-six patients from the validation cohort developed delayed high-grade AVB (all underwent successful PPI). In addition to differences in baseline clinical characteristics (Table 4), patients in the validation cohort had a higher D-PACE score compared to those in the derivation cohort (5 [IQR 4-7] vs 4 [IQR 2-5]; p<0.001) due to greater implantation depth (5.6 [IQR 4.0-7.5] mm vs 4.4 [IQR 3.5-5.3] mm; p<0.001), more frequent implantation of self-expanding valves (68.8% vs 27.6%; p<0.001) and greater next-day increase of the PR interval (0 [IQR –10 to +20] ms vs 0 [IQR –12 to +12] ms; p<0.001). However, there was no significant difference in the incidence of delayed AVB between the two cohorts (3.8% vs 5.8%; p=0.064). The D-PACE score was significantly correlated with the outcome of delayed high-grade AVB in the validation cohort, with an OR of 1.600 (95% CI: 1.375-1.860; p<0.001) for each increasing point. The area under the ROC curve in the validation cohort was 0.799 (95% CI: 0.730-0.868) (Figure 2), and the Hosmer-Lemeshow χ2 was 3.06 (p=0.880).
The performance of the score was unchanged in subgroups of the validation cohort that include only patients treated with current-generation valves or those from 2020 onwards (Supplementary Appendix 2, Supplementary Figure 3, Supplementary Figure 4).
Additionally, a second version of the score adapted for patients with atrial fibrillation/flutter in preprocedural and/or next-day ECG (D-PACE AF score) (Supplementary Table 4) was tested in a cohort of patients from groups A and B who were excluded from the derivation and validation cohorts (Supplementary Appendix 3, Supplementary Figure 5).
Table 4. Main characteristics of patients in the derivation and validation cohorts.
| Variables | Derivation cohort (n=915) | Validation cohort (n=936) | p-value |
|---|---|---|---|
| Age, years | 84 (81-87) | 81 (77-85) | <0.001 |
| Male sex | 42.5 | 56.5 | <0.001 |
| Prior myocardial infarction | 20.2 | 12.4 | <0.001 |
| Prior stroke | 5.9 | 4.4 | 0.140 |
| Prior TAVI or SAVR with sutureless bioprosthesis | 0.3 | 0.3 | 1.000 |
| Bicuspid aortic valve | 3.5 | 8.6 | <0.001 |
| STS score, % | 4.1 (2.9-6.1) | 3.4 (2.3-5.4) | <0.001 |
| EuroSCORE II, % | 3.8 (2.4-6.0) | 3.3 (2.1-4.9) | <0.001 |
| Estimated glomerular filtration rate, ml/min/1.73 m2 | 56 (41-72) | 57 (43-73) | 0.109 |
| Left ventricular ejection fraction <50% | 18.5 | 19.2 | 0.906 |
| Self-expanding valve | 27.6 | 68.8 | <0.001 |
| Implantation depth, mm | 4.4 (3.5-5.3) | 5.6 (4.0-7.5) | <0.001 |
| Transient procedural AVB | 2.5 | 2.8 | 0.834 |
| Preprocedural PR, ms | 178 (160-201) | 166 (152-200) | <0.001 |
| Preprocedural RBBB | 10.1 | 6.9 | 0.017 |
| Next-day PR variation^, ms | 0 (−12 to +12) | 0 (−10 to +20) | <0.001 |
| New-onset persistent† LBBB | 9.15 | 19.3 | <0.001 |
| New-onset persistent† RBBB | 0.7 | 1.6 | 0.088 |
| Late-onset high-grade AVB | 5.8 | 3.8 | 0.064 |
| D-PACE score | 4 (2-5) | 5 (4-7) | <0.001 |
| Values are expressed as median (interquartile range) or as %. ^Difference between values on next-day and preprocedural ECGs. †Conduction disturbances are considered persistent if they are present on both postprocedural and next-day ECGs. AVB: atrioventricular block; D-PACE: Delayed atrioventricular block Prediction for eArly disChargE; ECG: electrocardiogram; EuroSCORE: European System for Cardiac Operative Risk Evaluation; LBBB: left bundle branch block; RBBB: right bundle branch block; SAVR: surgical aortic valve replacement; STS: Society of Thoracic Surgeons; TAVI: transcatheter aortic valve implantation | |||
Discussion
The main findings of the study are as follows: (1) the incidence of delayed high-grade AVB requiring PPI more than 24 hours after TAVI is not negligible, as this event occurred in approximately 5% of the study population; (2) none of the patients undergoing valve-in-valve TAVI for conventional surgical valve degeneration developed late-onset high-grade AVB, suggesting a strong protective effect; (3) use of self-expanding valves, deeper valve implantation, longer preprocedural PR interval and a greater PR interval increase in next-day ECG, preprocedural RBBB, and persistent postprocedural LBBB and RBBB are independent predictors of delayed high-grade AVB; (4) the D-PACE score is an effective tool for risk stratification and can be used to help select patients who could be safely discharged 24 hours after the procedure.
Conduction abnormalities are common after TAVI, and they often trigger prolonged ECG monitoring and hospitalisation. In this setting, the identification of postprocedural PPI risk factors and implementation of standardised management algorithms for conduction disturbances represent very relevant progress11. Nevertheless, these protocols have not been universally adopted, and the fear of delayed (>24 h) high-grade AVB still represents a major limitation for early discharge in many centres and for many patients. Post-discharge continuous ECG monitoring has also been proposed for some patients19. Thus, identification of risk factors for high-grade AVB occurring after the first 24 hours may complement existing algorithms and facilitate patient selection for early discharge. In this regard, our study shows that the D-PACE score might represent a simple and useful tool for risk stratification of high-grade AVB and PPI 24 hours after the procedure.
Indeed, the D-PACE score could be integrated within a simple algorithm to stratify TAVI patients according to the risk of high-grade AVB occurring between 24 hours and 30 days after the procedure (Central illustration). As previously described, valve-in-valve patients with a conventional surgical valve have a negligible risk of late-onset AVB; they can therefore be classified as low risk and should be strongly considered for next-day discharge.
For the other patients, the next step is to calculate the D-PACE score, which in our study demonstrated a good discrimination ability in both the derivation and the validation cohorts (AUC 0.879 and 0.799, respectively). The multiparametric score is based on readily available procedural and electrocardiographic data and should be applied the day after the TAVI procedure: ECG variables are obtained from preprocedural and next-day (between 12 and 24 hours after the procedure) ECG tracings, while implantation depth is measured from angiograms obtained during the procedure. The score requires the PR interval to be measurable, so the algorithm cannot be applied to patients with atrial fibrillation/flutter on preprocedural and/or next-day ECGs.
Based on the score result, patients are classified into three categories: low, intermediate and high risk. The low-risk category includes patients with a score between 0 and 3: patients in this category have an estimated risk of 30-day high-grade AVB of less than 2%. These patients, who represent a significant portion of the study population (48.6% of the derivation cohort and 33.3% of the validation cohort), exhibited an extremely low incidence of late-onset AVB (less than 1% in both cohorts). Therefore, they represent a category suitable for next-day discharge without further ECG monitoring. Patients classified as high risk, with a D-PACE score ≥6, (between 21% in the derivation cohort and 38% in the validation cohort) showed a high incidence of delayed AVB, with rates between 8.7% (in the validation cohort) and 20.8% (in the derivation cohort). For this group of patients, additional ECG monitoring for a few days could help to identify most of the delayed AVBs, since almost two-thirds of the events occurred in the first four days after TAVI (Supplementary Figure 6). The remaining patients (around 30% in both cohorts) were classified as intermediate risk: the observed delayed AVB rates in the derivation and validation cohorts were 3.96% and 1.12%, respectively. Hence, early discharge can still be reasonably considered in this group, preferably with additional precautionary measures such as ambulatory ECG monitoring, which proved to be an effective strategy to rapidly identify delayed conduction disturbances in small cohorts of TAVI patients1920. Remarkably, in our patient population and in a similar group of prospectively followed patients21, no cases of sudden death or resuscitation were recorded, confirming the relative low risk of delayed high-grade AVBs. Although we did not document cases of sudden death in the first 30 days after TAVI, the cause of death was not known for 2 of the patients excluded from the study, due to a lack of clinical records.
A strength of this study is the systematic analysis of postprocedural and next-day ECG tracings. A relevant finding is that conduction disturbances which occur after the procedure but resolve within the first 24 hours do not affect the risk of delayed high-grade AVB. In contrast, new-onset bundle branch blocks and PR interval prolongation still present in next-day ECG are independent predictors of further atrioventricular conduction deterioration.
While new-onset LBBB after TAVI has been shown to be associated with PPI in several previous studies2223, this is, to our knowledge, the first time that new-onset RBBB has been identified as an independent predictor of high-grade AVB. Although only a few patients (0.7% of the derivation cohort) developed persistent RBBB after TAVI, the predictive power was even greater than that of new-onset LBBB. The right bundle branch is usually not damaged after TAVI due to the greater distance from the implanted valve, so its involvement can be considered a sign of very extensive damage to the conduction system, with a high risk of progression to complete AVB.
As mentioned above, none of the patients who underwent valve-in-valve TAVI for degeneration of a conventional surgical valve (130 patients in the entire study population) developed delayed AVB. This protective effect can be explained by the presence of the rigid ring of the surgical valve, which acts as a spacer between the percutaneous valve and the conduction system. In contrast, in valve-in-valve TAVI for degeneration of valves without a rigid ring (a group that includes percutaneous and sutureless surgical valves), this protective effect seems to be absent. In the derivation cohort, only 6 patients belonged to this group, yet 2 of them developed late-onset high-grade AVB. In addition, these valves have a portion of the frame inside the LVOT, close to the conduction system: this may be a risk factor for the occurrence of conduction disturbances, as the new transcatheter heart valve causes an overexpansion of the pre-existing bioprosthesis. However, this hypothesis needs to be confirmed in a larger population.
Limitations
First, this is an observational and mainly retrospective study, therefore, it is not immune to sources of bias.
Second, we did not assess computed tomography (CT) features, such as the volume and distribution of valvular and LVOT calcium and membranous septum length, which have been identified in previous studies2425 as relevant predictors of conduction disturbances after TAVI. Unfortunately, CT analysis would have significantly reduced the size of the study population, since old CT scans were unavailable in a significant number of patients. Similarly, the role of post-TAVI high-frequency atrial pacing to establish the Wenckebach point, a strategy that previously showed high negative predictive value for PPI26, was not evaluated in the current study. Future studies, prospectively including these parameters, might indeed help to refine the accuracy of available scores and represent useful information to guide and improve clinical practice.
Third, calculation of the D-PACE score requires the patient to be in sinus rhythm before and after the procedure, so the algorithm is not applicable to patients in atrial fibrillation/flutter. The modified version of the score (D-PACE AF score), which can be applied in this group of patients, requires further validation before a definite recommendation on its use can be made.
Fourth, measurement of implant depth using different angiographic projections (3-cusp or cusp-overlap view) in different patients may have introduced a small amount of variability into the measurement.
Last, most of the patients included in the study were treated with Evolut (Medtronic) or SAPIEN (Edwards Lifesciences) valves (Supplementary Table 1); consequently, the performance of the D-PACE score is more clearly demonstrated in patients treated with those devices. Its performance may be less robust in patients treated with other valves, which were underrepresented in the study.
Conclusions
The study confirms that high-grade AVB occurring more than 24 hours after TAVI is not a rare event, and its risk should be taken into account when considering early discharge after the procedure. The D-PACE score and algorithm is a simple tool to predict the occurrence of high-grade AVB between 24 hours and 30 days after TAVI, thus being potentially helpful to select patients for early discharge without compromising safety.
Impact on daily practice
The Delayed atrioventricular block Prediction for eArly disChargE (D-PACE) score, consisting of procedural and electrocardiographic variables, can be used the day after transcatheter aortic valve implantation to stratify patients according to the risk of delayed high-grade atrioventricular block. Based on the result of the score, it is possible to identify patients who can be safely discharged 24 hours after the procedure.
Conflict of interest statement
F. Saia has received consulting and lecture fees from Abbott, Edwards Lifesciences, and Medtronic. T. Palmerini has received speaker fees from Edwards Lifesciences and Medtronic. The other authors have no conflicts of interest regarding the content of the study to declare.
Supplementary data
To read the full content of this article, please download the PDF.

