Abstract
BACKGROUND: It has been suggested that coronary microvascular function decreases with age, irrespective of the presence of epicardial atherosclerosis.
AIMS: Our aim is to quantitatively investigate the effects of age on microvascular function in patients with normal coronary arteries.
METHODS: In 314 patients with angina with no obstructive coronary artery disease (ANOCA), microcirculatory function was tested using the continuous thermodilution method. In 305 patients, the association between age and both resting and hyperaemic myocardial blood flow (Q), microvascular resistance (Rμ), absolute coronary flow reserve (CFR) and microvascular resistance reserve (MRR) was assessed. In addition, patients were divided into 3 groups to test for differences based on age quartiles (≤52 years [24.9%], 53-64 years [49.2%], ≥65 years [25.9%]).
RESULTS: The mean age was 59±9 years with a range from 22 to 79 years. The mean resting Q (Qrest) was not different in the 3 age groups (88±34 mL/min, 82±29 mL/min, and 86±38 mL/min, R2=0.001; p=0.62). A trend towards a decreasing mean hyperaemic Q (Qmax) was observed with increasing age (223±79 mL/min, 209±84 mL/min, 200±80 mL/min, R2=0.010; p=0.083). The mean resting Rμ (Rμ,rest) were 1,204±460 Wood units (WU), 1,260±411 WU, and 1,289±455 WU (p=0.23). The mean hyperaemic Rμ (Rμ,hyp) increased significantly with advancing age (429±149 WU, 464±164 WU, 503±162 WU, R2=0.026; p=0.005).
Consequently, MRR decreased with age (3.2±1.2, 3.1±1.0, 2.9±0.9; p=0.038). This trend was present in both the patients with (n=121) and without (n=184) coronary microvascular dysfunction (CMD).
CONCLUSIONS: There is an age-dependent physiological increase in minimal microvascular resistance and decrease in microvascular function, which is represented by a decreased MRR and is independent of atherosclerosis. The age-dependent decrease in MRR was present in both patients with and without CMD and was most evident in patients with smooth coronary arteries.
Since the proportion of elderly patients presenting with angina is increasing, the impact of advancing age on health, including coronary (patho)physiology, deserves attention. The potential implication of this demographic trend on clinical decision-making is yet to be clarified, as are elderly-specific pathophysiological conditions and possible targeted therapeutics. Several age-dependent mechanisms have been identified linking ageing to loss of microcirculatory plasticity, adaptation and regeneration, leading to microvascular dysfunction and provoking susceptibility to ischaemia and angina1. However, these “physiological” effects of age on microvascular function have never been directly quantified in patients with no obstructive coronary arteries.
Previous studies have focused on patients with angina and obstructive coronary artery disease (CAD) and the implications of ageing on the development or progression of epicardial disease. In these patients, an age-dependent increase in microvascular resistance has been suggested. However, this is potentially confounded by the increasing severity of CAD with increasing age.
Microvascular function can be assessed by coronary flow and resistance measurements. Until recently, it was only possible to assess surrogate rather than absolute measures of coronary blood flow, using bolus thermodilution or Doppler flow methods, both with their intrinsic limitations2. Currently, direct quantification of true (absolute) coronary blood flow (Q) and microvascular resistance (Rμ) at rest (Qrest, Rμ,rest) and at hyperaemia (Qmax, Rμ,hyp) is possible using continuous thermoÂdilution23. This method has been shown to be safe and highly reproducible45, and its indices have been shown to correlate well with the flow derived from positron emission tomography (PET)-computed tomography (CT) and angina severity678. In addition, continuous thermodilution-based microvascular resistance reserve (MRR) is a novel index that is independent of epicardial resistance to flow and thus truly specific to the microvasculature9. This study sought to determine the influence of ageing on microvascular function in normal coronary arteries.
Methods
STUDY POPULATION
To select a population with normal coronary arteries, patients with angina with no obstructive coronary artery disease (ANOCA) who were referred for clinically indicated invasive coronary function testing (CFT) were included. Obstructive CAD had been ruled out prior to the CFT by CT or angiography. Consecutive patients from the Radboud University Medical Center (Nijmegen), Catharina Hospital (Eindhoven), and Maasstad Hospital (Rotterdam) were included in the Dutch prospective registry (NL-CFT). The rationale and design of the NL-CFT has been described previously10. Written informed consent was obtained from all patients, and approval by the local medical ethics committee was obtained.
STUDY DESIGN
All patients underwent continuous thermodilution measurements as part of complete CFT. CFT was performed in the left anterior descending artery (LAD) according to standard clinical practice, as described previously11. First, diagnostic angiography was performed to confirm the absence of significant obstructive CAD. Only patients with mild lesions (visually estimated at a <30% obstruction) were included. After spasm provocation testing using intracoronary acetylÂÂcholine injections, a sensor-tipped pressure guidewire was used to measure intracoronary pressure and flow. Next, as part of the standardised protocol, bolus thermodilution was performed, during which hyperaemia was induced with intravenous adenosine. As proximal and distal pressures were measured continuously, fractional flow reserve (FFR) was automatically calculated. If the FFR was positive, for example with mild but diffuse epicardial disease, the patient was excluded.
Continuous thermodilution was used to assess Q and R at rest and at hyperaemia, as well as MRR and absolute coronary flow reserve (CFR), on which we elaborate further in the next paragraph7. A dedicated, rapid-exchange coronary infusion microcatheter (RayFlow [Hexacath]) was advanced over the pressure guidewire into the proximal segment of the LAD. The pressure/temperature sensor was then positioned approximately 6 cm distal from the tip of the RayFlow catheter. To assess baseline Qrest and Rμ,rest, a slow infusion of saline (10 mL/min) at room temperature was started using an automated infusion system. After obtaining a steady-state mixing temperature (T), the wire was pulled back to the tip of the infusion catheter to assess the temperature of the infused saline (Ti). To reach Qmax and Rμ,hyp, the same protocol was repeated with a saline infusion at 20 mL/min, as described previously12.
Absolute coronary flow (Q) (in mL/min) can be calculated, using the following formula13:
(formule 1)
Where Qi is the infusion rate of saline from the infusion pump (10 mL/min at rest and 20 mL/min at hyperaemia for the LAD), Ti is the temperature of the infused saline when it exits the infusion catheter, and T is the temperature of the mixture of blood and saline in the distal part of the coronary artery during steady-state infusion. The correction factor 1.08 compensates for the difference in specific heat and density between saline and blood14.
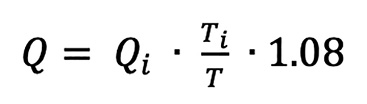
formule 1.
ASSESSMENT OF MICROVASCULAR FUNCTION USING INTRACORONARY PHYSIOLOGY
Using the pressure and absolute flow measurements at rest and at hyperaemia (Qrest and Qmax), absolute CFR and MRR were calculated. CFR is the ratio of hyperaemic coronary blood flow to the resting coronary blood flow (Qmax/Qrest). This has classically been used to represent the microvascular vasodilatory capacity and has extensively been demonstrated to be an important predictor of worse prognosis15. However, coronary blood flow is dependent on epicardial disease and, as such, is not specific to the microcirculation. Therefore, MRR was recently introduced by de Bruyne et al, who demonstrated that it is independent of epicardial disease, variation in driving pressure between resting and hyperaemic measurements, and myocardial mass9.
(formule 2)
MRR can be calculated as above, where the first term is classical CFR, the second term compensates for epicardial disease (inverse value of FFR) and the third term for haemodynamic variations. The equation can also be rewritten as follows:
(formule 3)
All calculations were performed by dedicated software (Coroventis CoroFlow [Abbott]).
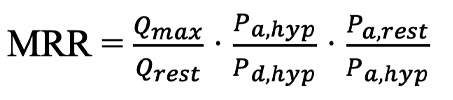
formule 2.
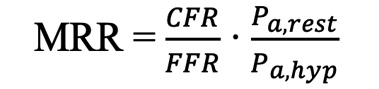
formule 3.
RATIONALE OF A MICROVASCULAR RESISTANCE RESERVE CUTOFF
We used an MRR value of ≤2.7 to define abnormal microvascular function, as demonstrated by previous evidence published by our group16. A higher cutoff of 3.0 was suggested by other authors using bolus thermodilution measurements17. However, as bolus thermodilution measurements tend to overestimate continuous thermodilution-derived MRR8, the value of 2.7 was chosen for this study.
STATISTICAL ANALYSES
The normality and homogeneity of the variances were assessed using histograms. Continuous variables are presented as mean±standard deviation (SD) or as median (interquartile range [IQR]) where appropriate. Categorical variables are presented as counts (percentage). For descriptive statistics, the study population was stratified into 3 representative age categories, defined by the quartile of age. Patients within the first quartile (≤52 years) were considered “young”, patients across quartiles 2 and 3 (>52 and <65 years) “intermediate”, and patients within the fourth quartile (≥65 years) “elderly” (Central illustration). Overall differences between groups were compared with the chi-square test, followed by post hoc Student’s t-tests or Mann-Whitney U tests.
Linear regression (analysis of variance [ANOVA] test) was used to compare the physiological indices between the 3 age groups. The correlation between indices was estimated by calculating the Pearson correlation coefficient (Ï). Backward regression with linear mixed models was used to identify independent predictors of resting Rμ, hyperaemic Rμ and MRR with candidate variables, which included all baseline clinical characteristics, as presented in Table 1. These results are presented as beta±standard error (SE) and standardised coefficients to facilitate comparison. A two-sided p-value<0.05 was considered statistically significant. Analyses were performed with SPSS Statistics (IBM) and Prism 7 (GraphPad).
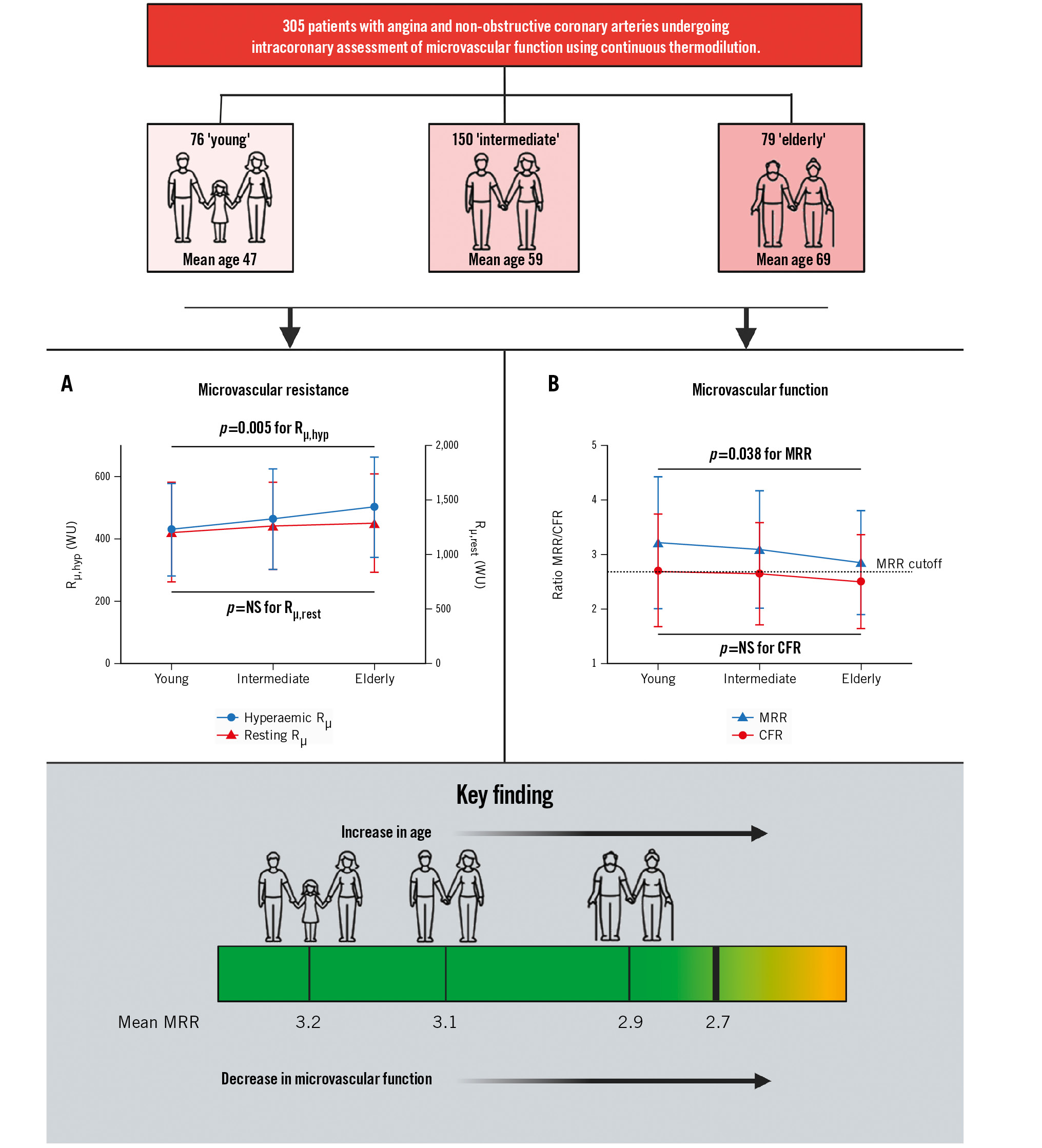
Central illustration. Effects of age on microvascular function in patients with normal coronary arteries. A) The mean microvascular resistance during hyperaemia according to age group. Rµ,hyp increases as patients age, while Rµ,rest remains similar with advancing age. B) The mean MRR and CFR according to age group. The MRR decreases as patients with normal coronary arteries age, but the CFR does not significantly decrease in these patients. CFR: coronary flow reserve; MRR: microvascular resistance reserve; NS: not significant; Rµ,hyp: hyperaemic absolute microvascular resistance; Rµ,rest: resting absolute microvascular resistance; WU: Wood units
Table 1. Clinical characteristics.
| Clinical characteristics | n=305 |
|---|---|
| Age, years | 59±9 |
| Female | 251 (83) |
| Hypertension | 149 (49) |
| Dyslipidaemia | 131 (43) |
| Diabetes | 32 (11) |
| Family history | 140 (47) |
| Current smoker | 28 (10) |
| History of autoimmune disease | 37 (12) |
| Data are mean±SD or n (%). SD: standard deviation | |
Results
CLINICAL AND PROCEDURAL CHARACTERISTICS
A total of 314 consecutive patients were investigated between February 2020 and November 2022. The clinical characteristics of the complete study population are shown in Table 1. In total, 261 (83%) were female. Nine patients were excluded from the analysis: in 1 patient, hyperaemic absolute flow measurements could not be performed because of a transient atrioventricular block; 1 patient had significant epicardial wire spasm in the LAD during continuous thermodilution; and 7 patients were excluded because of functionally significant obstructive CAD, based on an FFR below 0.80 during the bolus thermodilution measurements.
The mean age of the population was 59±9 years, and this ranged between 22 and 79. Patients were stratified based on their age quartiles into “young” (≤52 years; n=79 [25%]), “intermediate” (>52 and <65 years of age; n=152 [49%]), and “elderly” (≥65 years; n=83 [26%]). Non-obstructive CAD in the LAD was confirmed in all remaining patients, and the mean FFR was 0.90±0.05 and similar across the three groups (0.90±0.04, 0.90±0.05, 0.90±0.04; p=0.66).
Clinical and procedural characteristics for each age category are shown in Table 2. Classical risk factors for cardiovascular disease were generally less prevalent in younger patients, who had less history of hypertension and dyslipidaemia.
Table 2. Clinical and procedural characteristics.
| Age | p-value | |||
|---|---|---|---|---|
| Young (n=76) | Intermediate (n=150) | Elderly (n=79) | ||
| Clinical characteristics | ||||
| Age, years | 47±5 | 59±3 | 69±4 | 0.001 |
| Female | 63 (83) | 128 (84) | 65 (83) | 0.94 |
| Hypertension | 26 (33) | 79 (52) | 49 (59) | 0.004 |
| Dyslipidaemia | 23 (29) | 71 (47) | 42 (51) | 0.013 |
| Diabetes | 5 (6) | 14 (9) | 15 (18) | 0.050 |
| Family history | 33 (42) | 73 (49) | 38 (46) | 0.63 |
| Current smoker | 13 (17) | 11 (8) | 6 (7) | 0.06 |
| History of autoimmune disease | 7 (9) | 24 (16) | 6 (98) | 0.12 |
| Angiographic characteristics | ||||
| FFR | 0.89±0.05 | 0.89±0.07 | 0.90±0.05 | 0.74 |
| RFR* | 0.93±0.03 | 0.93±0.03 | 0.92±0.03 | 0.68 |
| Continuous thermodilution | ||||
| Qrest, mL/min | 88±34 | 82±29 | 86±38 | 0.62 |
| Qmax, mL/min | 223±79 | 209±84 | 200±80 | 0.083 |
| CFR | 2.7±1.0 | 2.7±0.9 | 2.5±0.9 | 0.18 |
| Rµ,rest, WU | 1,204±460 | 1,263±406 | 1,289±456 | 0.23 |
| Rµ,hyp, WU | 429±149 | 464±164 | 503±162 | 0.005 |
| MRR | 3.2±1.2 | 3.1±1.1 | 2.9±1.0 | 0.038 |
| Prevalence of CMD – MRR <2.7 | 28 (37) | 57 (38) | 36 (46) | 0.45 |
| Data are mean±SD or n (%). *available for 277 patients. p-values in bold indicate statistical significance. CFR: coronary flow reserve; CMD: coronary microvascular dysfunction; FFR: fractional flow reserve; MRR: microvascular resistance reserve; Qmax: hyperaemic absolute coronary flow; Qrest: resting absolute coronary flow; RFR: resting full-cycle ratio; Rµ,hyp: hyperaemic absolute microvascular resistance; Rµ,rest: resting absolute microvascular resistance; SD: standard deviation; WU: Wood units | ||||
INFLUENCE OF AGE ON ABSOLUTE CORONARY FLOW, ABSOLUTE MICROVASCULAR RESISTANCE, CFR AND MRR
The mean Qrest was not correlated with age (p=0.044; p=0.45) and was not different between the groups (88±34 mL/min, 82±29 mL/min, and 86±38 mL/min, R2=0.001; p=0.62). The mean Qmax was significantly correlated with age (p=0.113; p=0.048); additionally, a trend towards a decreasing average Qmax per group was observed with increasing age (223±79 mL/min, 209±84 mL/min, 200±80 mL/min, R2=0.010; p=0.083) (Figure 1). This difference in Qmax but not Qrest did lead to a numerical but nonsignificant difference in continuous thermoÂdilution-based CFR between the groups (2.7±1.0, 2.7±0.9, 2.5±0.9, R2=0.006; p=0.18) (for correlation: p=0.076, p=0.19) (Central illustration B).
The mean Rμ,rest for each group was 1,204±460 WU, 1,260±411 WU and 1,289±455 WU (p=0.23) and did not correlate with age (p=0.070; p=0.23). The mean Rμ,hyp, or minimal microvascular resistance, did significantly correlate with age (p=0.154; p=0.007) and increased with advancing age (429±149 WU, 464±164 WU, 503±162 WU, R2=0.026; p=0.005) (Central illustration A).
Consequently, MRR also correlated with age (p=0.114; p=0.046) and decreased with increasing age (3.2±1.2, 3.1±1.1, 2.9±1.0, R2=0.014; p=0.038) (Central illustration B).
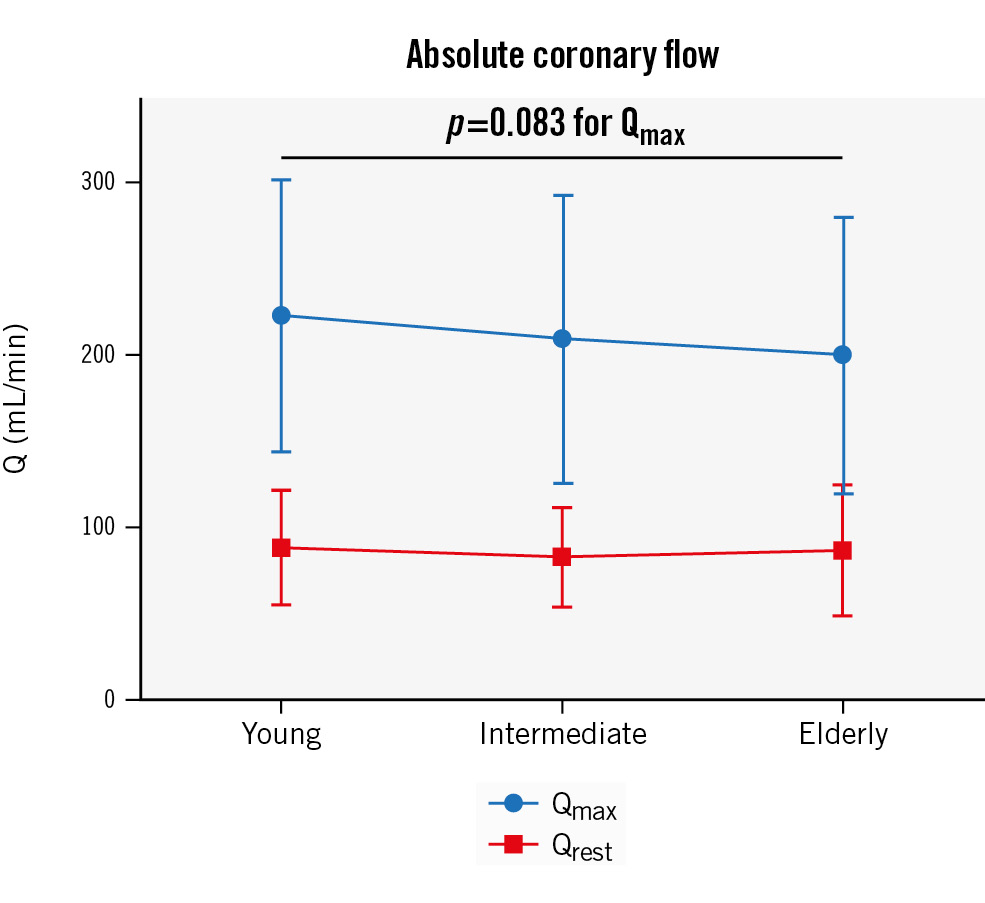
Figure 1. Mean absolute coronary flow (Q) at rest and during hyperaemia according to age group. Resting absolute coronary flow (Qrest) remains the same with advancing age, but hyperaemic absolute coronary flow (Qmax) numerically reduces with ageing.
INFLUENCE OF AGE ON MICROVASCULAR FUNCTION IN PATIENTS WITH AND WITHOUT CMD
Based on an MRR cutoff value of 2.7, 121 patients had CMD and 184 had no CMD (normal microvascular function), with the highest prevalence of CMD in the elderly group (Figure 2). There was no significant difference in the mean age between patients with and without CMD (59±9 years and 58±9 years; p=0.35, respectively). The intracoronary physiological measures in the different age groups in patients with and without CMD are presented in Table 3. In the non-CMD group, age was still correlated with MRR (Ï=−0.150; p=0.042) and demonstrated a decreasing trend with ageing (3.9±1.0, 3.7±0.9, 3.5±0.7; p=0.067) (Figure 3). Furthermore, patients with CMD did not show a decrease in MRR with ageing (2.1±0.3, 2.1±0.4, 2.0±0.4; p=0.71), but they did demonstrate a significant increase in Rμ,hyp (460±126 WU, 538±184 WU, 551±168 WU; p=0.040) (Figure 3).
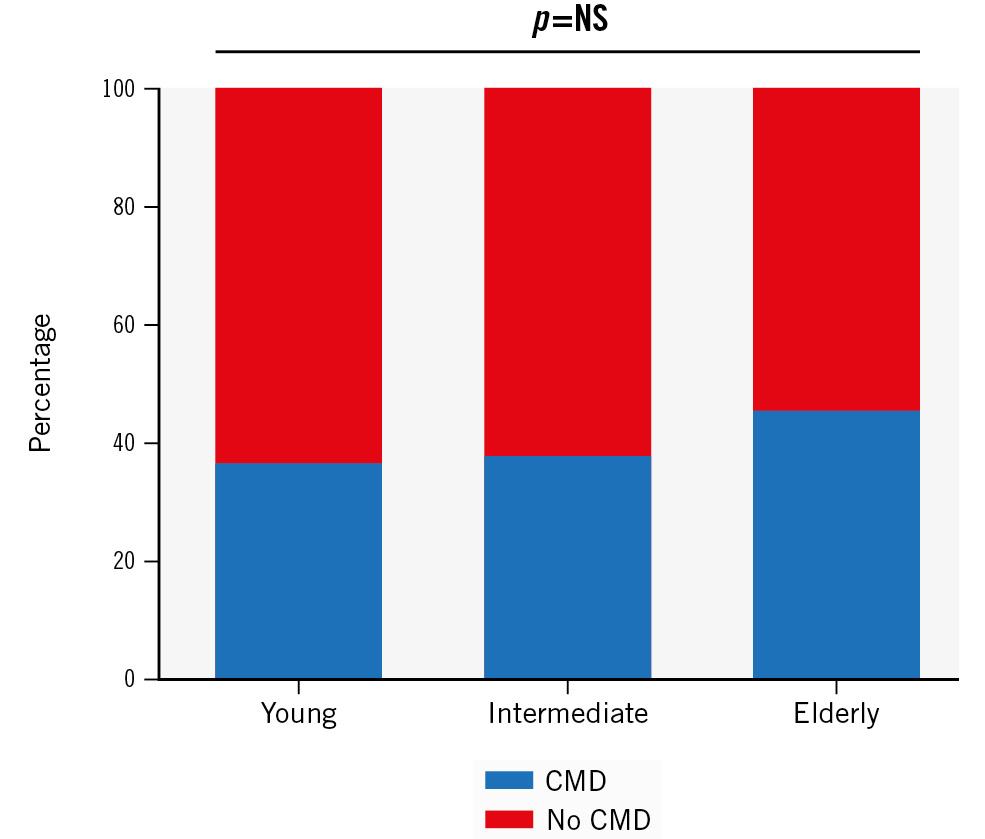
Figure 2. Prevalence of CMD according to age group. The prevalence of CMD (based on an MRR cutoff of 2.7) in ANOCA patients numerically increases with advancing age. ANOCA: angina with no obstructive coronary artery disease; CMD: coronary microvascular dysfunction; MRR: microvascular resistance reserve; NS: not significant
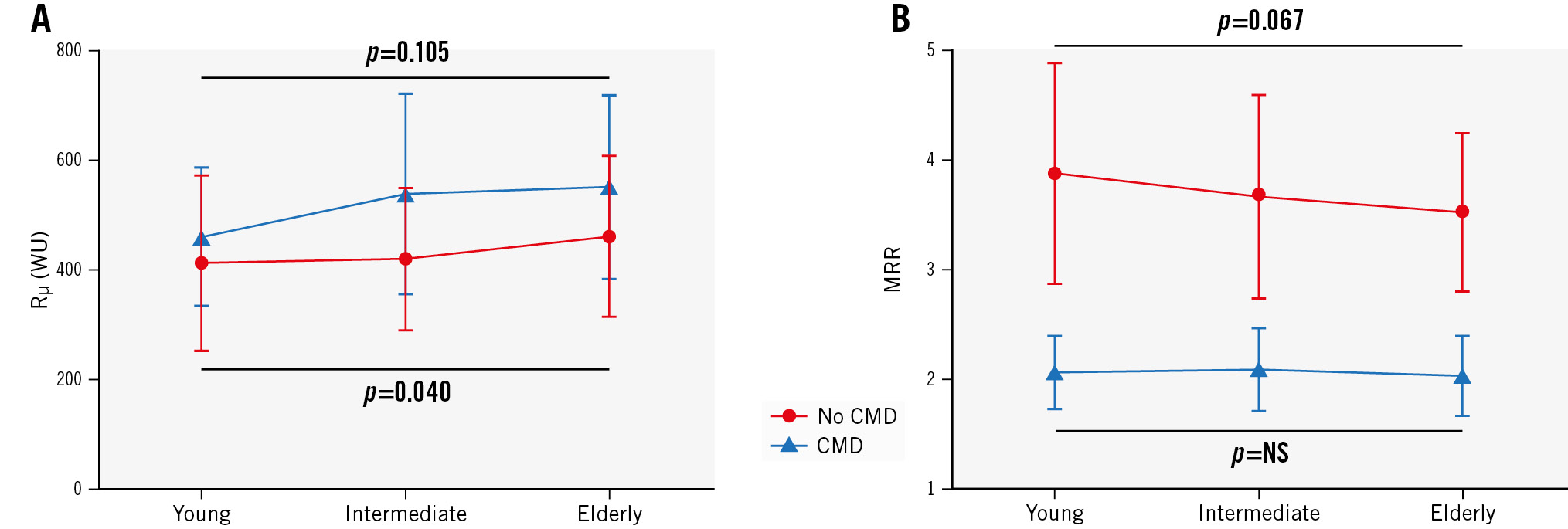
Figure 3. Trends of Rµ,hyp and MRR in patients with and without CMD. A) Patients with no CMD (normal coronaries) demonstrate a significant increase in Rµ,hyp, whereas patients with CMD only show a numerical increase. B) In the patients with no CMD, MRR demonstrates a decreasing trend with ageing. Patients with CMD demonstrate equal MRR values in the 3 age groups. CMD: coronary microvascular dysfunction; MRR: microvascular resistance reserve; NS: not significant; Rµ,hyp: hyperaemic absolute microvascular resistance; WU: Wood units
INFLUENCE OF AGE ON MICROVASCULAR FUNCTION IN PATIENTS WITH SMOOTH CORONARY ARTERIES VERSUS NON-OBSTRUCTIVE ATHEROSCLEROTIC CORONARY ARTERIES
In an additional analysis, we evaluated the differences in the influence of age on microvascular function in patients with angiographically smooth coronary arteries (47%) versus patients with any epicardial atherosclerotic irregularity on angiography (53%).
As displayed in Table 4, MRR significantly decreased with advancing age in patients with smooth coronary arteries (3.4±1.2, 3.0±1.0, 2.6±0.7; p=0.004) but not in patients with any epicardial atherosclerosis (3.0±1.2, 3.2±1.1, 3.0±1.0; p=0.61). However, Rμ,hyp significantly increased in both groups (438±157 WU, 481±153 WU, 529±144 WU; p=0.017 and 460±126 WU, 538±184 WU, 551±168 WU; p=0.04, respectively).
Table 4. Physiological indices of microvascular function according to the different age groups of patients with smooth coronary arteries versus patients with any epicardial atherosclerotic irregularity.
| Smooth coronary arteries (47%) | Any atherosclerotic irregularity/obstruction (53%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Young (n=46) | Intermediate (n=70) | Elderly (n=24) | p-value | Young (n=26) | Intermediate (n=75) | Elderly (n=54) | p-value | |
| Age, years | 46 (6) | 59 (3) | 69 (4) | 0.001 | 48 (3) | 59 (3) | 69 (4) | 0.001 |
| Qrest, mL/min | 84 (31) | 81 (28) | 87 (39) | 0.87 | 113 (35) | 99 (41) | 105 (42) | 0.46 |
| Qmax, mL/min | 221 (75) | 205 (77) | 194 (73) | 0.14 | 199 (55) | 178 (67) | 186 (75) | 0.49 |
| CFR | 2.8 (1.0) | 2.7 (0.9) | 2.4 (0.7) | 0.085 | 2.6 (1.1) | 2.7 (1.0) | 2.6 (0.9) | 0.64 |
| Rµ,rest, WU | 1,266 (460) | 1,311 (417) | 1,280 (442) | 0.81 | 920 (301) | 1,070 (346) | 1,064 (363) | 0.12 |
| Rµ,hyp, WU | 438 (157) | 481 (153) | 529 (144) | 0.017 | 460 (126) | 538 (184) | 551 (168) | 0.04 |
| MRR | 3.4 (1.2) | 3.0 (1.0) | 2.6 (0.7) | 0.004 | 3.0 (1.2) | 3.2 (1.1) | 3.0 (1.0) | 0.61 |
| Data are presented as mean (SD). p-values in bold indicate statistical significance. CFR: coronary flow reserve; MRR: microvascular resistance reserve; Qmax: hyperaemic absolute coronary flow; Qrest: resting absolute coronary flow; Rµ,hyp: hyperaemic absolute microvascular resistance; Rµ,rest: resting absolute microvascular resistance; WU: Wood units | ||||||||
MULTIVARIABLE ANALYSIS
The best multivariable model for the prediction of MRR (R2=0.050; p=0.002) included age, sex and a diagnosed autoimmune disorder (Table 5). For Rμ,hyp, only age, sex and a history of diabetes were identified as predictors (R2=0.046; p=0.004). After correction for these variables, age was the strongest independent predictor for both MRR and Rμ,hyp. For Rμ,rest, only a history of hypertension was identified as an independent predictor (R2=0.015; p=0.039).
Table 5. Best-fit multivariable linear regression model for the prediction of MRR, Rµ,hyp and Rµ,rest.
| Beta | SE | Standard coefficient | p-value | |
|---|---|---|---|---|
| MRR | ||||
| Age | −0.014 | 0.007 | −0.111 | 0.055 |
| Sex | 0.453 | 0.170 | 0.154 | 0.008 |
| Diagnosed AI disorder | 0.409 | 0.192 | 0.123 | 0.034 |
| Rµ,hyp | ||||
| Age | 2.55 | 1.061 | 0.140 | 0.017 |
| Sex | −46.4 | 24.9 | −0.108 | 0.063 |
| History of DM | 27.5 | 15.7 | 0.101 | 0.082 |
| Rµ,rest | ||||
| History of hypertension | 75.4 | 36.4 | 0.121 | 0.039 |
| Results of backward regression with linear mixed models to identify independent predictors of resting Rµ, hyperaemic Rµ and MRR. All baseline clinical characteristics, as presented in Table 1, are used in the model. AI: autoimmune; DM: diabetes mellitus; MRR: microvascular resistance reserve; Rµ: microvascular resistance; Rµ,hyp: hyperaemic absolute microvascular resistance; Rµ,rest: resting absolute microvascular resistance; SE: standard error | ||||
Discussion
We elaborate further on the following three main findings: 1. The current study provides evidence that coronary microÂvascular senescence exists and is expressed as a decrease in microvascular function. This is driven by an increase in minimal microvascular resistance, which leads to a decreased maximal coronary blood flow and decrease in MRR (Central illustration).
2. The physiological link between ageing and decrease in microvascular function has been previously suggested but was difficult to confirm and quantify, since resistance measurements that were independent of epicardial resistance, and thus specific for the microvasculature, were unavailable.
3. The decrease in MRR with increasing age was present in both patients with and without CMD and most evident in patients with smooth coronary arteries.
These findings are important when considering global demographic changes, where an increasing number of elderly patients are referred to the coronary catheterisation laboratory with chest pain syndromes.
PREVIOUS STUDIES EVALUATING THE EFFECT OF AGEING ON MICROCIRCULATORY FUNCTION
Several studies have investigated the influence of ageing on the coronary microcirculation. However, these studies have been performed in patients with suspected obstructive CAD using surrogates of coronary blood flow181920. We emphasise that the presence of (hidden or overt) atherosclerotic disease is an important confounder in these analyses, with obstructive coronary artery disease already consuming part of the vasodilatory reserve of the microcirculation.
A study by van de Hoef et al investigated 228 patients with either Doppler or bolus thermodilution measurements to assess (surrogates of) coronary haemodynamics according to age20. They showed that age is associated with a progressive increase in minimal microvascular resistance in the entire myocardium, leading to an impairment of coronary vasodilatory reserve. In a subselection of patients, additional measurements in non-obstructed vessels (n=172) were performed. Interestingly, in these non-obstructed vessels, the same pattern was seen as in our cohort: with advancing age, CFR decreases because of an increase in hyperaemic resistance (but not resting resistance). Furthermore, they found that in the absence of obstructive CAD, age was the strongest independent predictor of CFR, followed by a history of dyslipidaemia.
There are also previous studies reporting on the effect of ageing on myocardial blood flow as assessed non-invasively by PET in the non-obstructive coronary arteries of healthy volunteers. However, their results are contradictory212223. Our study is the first to use intracoronary measurements of absolute coronary volume flow in normal coronary arteries, confirming an association between increasing age and decreased microvascular function.
THE PHYSIOLOGICAL EFFECT OF AGEING ON THE MICROCIRCULATORY FUNCTION IN PATIENTS WITH NORMAL CORONARY ARTERIES
For years, several (patho)physiological processes on microcirculatory function instigated by ageing have been hypothesised and identified. Recently, LeBlanc et al1 reviewed the available data on these processes and even pointed out that targeting these processes might lead to improved microvascular function. However, in vivo confirmation was lacking. In summary, they describe that in ageing, factors establishing vessel function and structure (such as nitric oxide [NO] bioavailability, hydrogen peroxide [H2O2] utilisation, oxidative stress, diastolic filling, angiogenesis and inflammation) become unbalanced. This predisposes the ageing heart and vasculature to injury, promotes maladaptation of the cardiovascular system, and limits recovery from ischaemic events. As a result, adaptation of the coronary microcirculation to both physiological and pathophysiological challenges is hampered, eventually compromising flow capacity1. The results of our study in patients with normal coronaries confirm this hypothesis.
Limitations
First, although non-obstructive coronary arteries were investigated exclusively, all patients presented with angina. These patients have a high prevalence of microcirculatory dysfunction. This might have led to an overestimation of the true effect of ageing on the microcirculatory function. However, because a large invasive study in volunteers is not ethical, we believe that our population is the best possible representation of normal. Furthermore, in a subanalysis of patients without pre-existing CMD, the correlation between MRR and ageing remained.
Second, the majority of patients were female (83%). As ANOCA is a more frequent condition in females24, a referral bias for CFT might exist. A previous study from our group demonstrated that bolus thermodilution-derived CFR was lower in females, primarily due to an increased resting flow25. This was similar in our cohort where MRR was significantly lower in females (data not shown), and sex proved to be a significant predictor of MRR (Table 4). However, the females were equally distributed between the age groups (83%, 84% and 82%, respectively), so this will not have influenced the effect of ageing on MRR. Furthermore, the microvascular function pattern was not different when analysed in male patients.
Third, due to the exclusion of all patients with significant epicardial disease, the influence of an age-related increase of epicardial disease was not accounted for. However, when evaluating the differences in smooth coronary arteries versus any epicardial atherosclerotic burden, we found that the effect of ageing on MRR was particularly strong in patients with smooth coronary arteries. This underlines that there is an effect of ageing on microvascular function independent of atherosclerosis.
Lastly, in this study, the MRR was used as the most important index of coronary microvascular function. However, since this index was only recently introduced, it has to be validated more extensively to ensure its use as an accurate surrogate of microvascular function and prognosis1617.
Table 4. Physiological indices of microvascular function according to the different age groups of patients with smooth coronary arteries versus patients with any epicardial atherosclerotic irregularity.
| Smooth coronary arteries (47%) | Any atherosclerotic irregularity/obstruction (53%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Young (n=46) | Intermediate (n=70) | Elderly (n=24) | p-value | Young (n=26) | Intermediate (n=75) | Elderly (n=54) | p-value | |
| Age, years | 46 (6) | 59 (3) | 69 (4) | 0.001 | 48 (3) | 59 (3) | 69 (4) | 0.001 |
| Qrest, mL/min | 84 (31) | 81 (28) | 87 (39) | 0.87 | 113 (35) | 99 (41) | 105 (42) | 0.46 |
| Qmax, mL/min | 221 (75) | 205 (77) | 194 (73) | 0.14 | 199 (55) | 178 (67) | 186 (75) | 0.49 |
| CFR | 2.8 (1.0) | 2.7 (0.9) | 2.4 (0.7) | 0.085 | 2.6 (1.1) | 2.7 (1.0) | 2.6 (0.9) | 0.64 |
| Rµ,rest, WU | 1,266 (460) | 1,311 (417) | 1,280 (442) | 0.81 | 920 (301) | 1,070 (346) | 1,064 (363) | 0.12 |
| Rµ,hyp, WU | 438 (157) | 481 (153) | 529 (144) | 0.017 | 460 (126) | 538 (184) | 551 (168) | 0.04 |
| MRR | 3.4 (1.2) | 3.0 (1.0) | 2.6 (0.7) | 0.004 | 3.0 (1.2) | 3.2 (1.1) | 3.0 (1.0) | 0.61 |
| Data are presented as mean (SD). p-values in bold indicate statistical significance. CFR: coronary flow reserve; MRR: microvascular resistance reserve; Qmax: hyperaemic absolute coronary flow; Qrest: resting absolute coronary flow; Rµ,hyp: hyperaemic absolute microvascular resistance; Rµ,rest: resting absolute microvascular resistance; WU: Wood units | ||||||||
Conclusions
There is an age-dependent physiological increase in hyperaemic microvascular resistance and decrease in microvascular function, as represented by a decreased MRR. This age-dependent decrease was present in both the patients with and without CMD and was particularly evident in patients with smooth coronary arteries, which underlines the independence of atherosclerosis.
Impact on daily practice
This study provides evidence of an age-dependent decrease in microvascular function that is independent of atherosclerosis. Microvascular dysfunction should be considered as an alternative diagnosis in elderly patients with no obstructive coronary artery disease. The clinical relevance and prognostic impact of this age-dependent decrease of microvascular function remains unknown. Future research is needed to investigate whether age-related coronary microvascular dysfunction is as pathological as non-age-related coronary microvascular dysfunction.
Funding
The NL-CFT registry is partly funded by the IMPRESS consortium.
Conflict of interest statement
P. Damman has received consultancy fees and research grants from Philips; he has received a research grant from Abbott. N. Pijls has received institutional research grants from Abbott and Hexacath; is a consultant for Abbott and Opsens Medical; has minor equities in Philips, ASML, HeartFlow and General Electric; is a member of the scientific advisory board of HeartFlow; and has patents pending on diagnostic methods for quantifying aortic valve stenosis and microvascular physiology. The other authors have no conflicts of interest to declare.

