Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Multilayer in-stent restenosis (ISR) remains a clinical challenge. Intravascular brachytherapy (IVBT) offers a “metal-free” treatment modality for multilayer drug-eluting stent (DES)-ISR; however, long-term outcome data on IVBT safety and efficacy are lacking.
Aims: We sought to compare 3-year clinical outcomes between patients treated with IVBT and those treated with a non-IVBT strategy.
Methods: Patients treated for multilayer DES-ISR (≥2 layers) at Mount Sinai Hospital (2012-2019) were included for analysis. The primary outcome was major adverse cardiac events (MACE), a composite of all-cause death, target lesion revascularisation and myocardial infarction, at 3-year follow-up.
Results: A total of 647 patients (mean age 66.6±9.9 years, 25.5% female) were included: 453 patients (70%) were treated with IVBT and 194 patients (30%) with a non-IVBT strategy. Baseline characteristics were similar, except for IVBT-treated patients having a higher incidence of prior coronary artery bypass grafting. The IVBT group had a lower mean SYNTAX score (11.9±10.7 vs 14.2±11.3; p=0.028) and were significantly less likely to receive a DES (0.4% vs 25.8%; p<0.001). At 3-year follow-up, the incidence of MACE was lower in the IVBT-treated group compared to the non-IVBT group (propensity score-adjusted analysis: 39.5% vs 47.8%; hazard ratio 0.73, 95% confidence interval: 0.53-0.99; p=0.044). There were no significant differences between the incidence of the individual components of MACE in each group.
Conclusions: Multilayer DES-ISR is associated with a high rate of adverse outcomes at 3-year follow-up. Treatment with IVBT was associated with a lower rate of MACE compared to treatment with a non-IVBT strategy at long-term follow-up.
Drug-eluting stents (DES) are associated with a significantly lower rate of in-stent restenosis (ISR) than bare metal stents (BMS), and today, DES implantation represents the standard of care for percutaneous coronary intervention (PCI)123. However, stent-related clinical events accrue at a yearly rate of at least 2-10%, even after DES implantation, and ISR consistently accounted for one out of ten PCI cases performed each year between 2009 and 2017 in the United States456. In addition, treating ISR by further DES implantation begets the risk of recurrent ISR (i.e., within two or more stent layers)13.
Treating multilayer ISR remains an unmet clinical need, and the optimal treatment strategy remains unclear. Regardless of management, adverse clinical outcomes are frequent during short-term follow-up3789. Implantation of a third stent layer has been associated with a progressively increased risk of adverse outcomes, and long-term patency after balloon angioplasty in this context is poor31011. Furthermore, in the United States, “metal-free” strategies for treating multilayer ISR have not progressed at the same rate as in other regions, such as Europe.
Against this background, there has been a resurgence of interest in intravascular brachytherapy (IVBT) as part of the armamentarium for the treatment of ISR in the United States, with IVBT use increasing from 0.1% of ISR cases in 2010 to 0.8% of cases in 20174. Nonetheless, the evidence underpinning PCI with IVBT is limited to small single-arm studies, with mostly short-term follow-up. In particular, the late “catch-up” phenomenon whereby the benefits of IVBT may be mitigated beyond 1 year has been poorly investigated. Thus, this study aims to compare the clinical outcomes of patients with multilayer ISR treated with an IVBT versus non-IVBT PCI strategy at 3-year follow-up.
Methods
Study design and patient population
The study population of this observational retrospective study included prospectively enrolled consecutive subjects who underwent PCI at a large tertiary hospital (Mount Sinai Hospital, New York, NY, USA) between 1 January 2012 and 31 December 2019. Patients undergoing PCI for DES-ISR were identified, and only patients with multilayer ISR (≥2 stent layers) were included. If the last layer of stent was a non-DES, the patient was excluded. Only the index procedure was included for patients who underwent multiple procedures meeting the inclusion criteria. Patients with both stable coronary artery disease and acute coronary syndrome were included. Exclusion criteria included a cardiogenic shock presentation and angiographic findings of stent thrombosis (ST). Institutional preference was to perform IVBT after lesion preparation when patients presented with multilayer ISR. However, the treatment strategy employed was ultimately at the discretion of the interventional cardiologist.
Patients were divided into two groups based on the PCI treatment strategy employed. Patients with multilayer ISR treated with an IVBT PCI strategy were compared to patients treated with a non-IVBT PCI strategy. After hospital discharge, patients were routinely reviewed at weeks 2 and 4 post-procedure. Following this, the frequency of follow-up was at the discretion of the patient’s cardiologist.
All patients provided written informed consent before PCI was performed, and the study was approved by the institutional review board at Mount Sinai Hospital, New York. The study complied with the Declaration of Helsinki and was approved by the institution’s ethics committee.
Data collection, follow-up, and event adjudication
The prospective Mount Sinai Hospital catheterisation laboratory database was analysed. Baseline patient and procedural characteristics are entered into this database during the index hospitalisation for PCI. Active follow-up was performed by dedicated research personnel through in-person visits, telephone interviews or mail and review of electronic medical records at 30-day and 1-year follow-ups after PCI, per local standard institutional follow-up protocol. For this study, research personnel conducted additional follow-up to collect 3-year follow-up data. In addition to contacting patients via telephone, supporting data were gleaned from hospital medical records, primary care physicians, and external death records (such as online obituaries and the Social Security Death Index). No angiographic follow-up was scheduled unless clinically indicated.
Device and procedure
PCI for multilayer ISR was performed according to contemporary best practice guidelines. Before commencing PCI, therapeutic anticoagulation (activated clotting time >300 s) was achieved with bivalirudin or unfractionated heparin. Lesion preparation was performed using non-compliant balloons, speciality balloons (i.e., cutting balloons), and/or atherectomy (i.e., rotational, orbital, or laser atherectomy) according to lesion morphology and operator preference. The need for atherectomy was predominately guided by angiographic evidence of calcification. Atherectomy was also considered when there was residual stenosis (>30%) despite lesion modification with percutaneous transluminal coronary angioplasty or atherotomy.
Patients in the IVBT arm received localised radiation using the Novoste Beta-Cath 3.5F System (Best Vascular) using a strontium-90/yttrium-90 isotope. This radioactive isotope produces β particles and has a half-life of 28.8 years and energies up to 2.27 MeV. Radiation was delivered using a triple-lumen closed-end catheter with radiopaque markers such that the source covered at least 5 mm on either side of the lesion. Source lengths of 40 mm or 60 mm were used based on the target lesion length. Radiation dosage was determined based on the diameter of the vessel (angiographically assessed in the majority of cases), which ranged from 18.4 to 23 Gy from the centre of the source. Dwell time, calculated based on lesion length and vessel diameter, usually ranged between 200 and 300 seconds. The source was retracted at the end of the IVBT procedure, and the catheter was removed from the body. After the procedure, a room survey was performed to confirm that no isotope remained outside the catheter. A radiation oncologist oversaw the entire procedure following Nuclear Regulatory Commission guidelines12. For patients in the non-IVBT group (control), standard practice for PCI was followed, and the decision to place a further layer of stent was made at the discretion of the operator. Placement of a DES in the IVBT arm was reserved for significant dissections caused by lesion preparation. After the procedure, patients in the control group received dual antiplatelet therapy (DAPT) with aspirin and a thienopyridine agent for 12 months, followed by aspirin monotherapy. For patients treated with IVBT at our institution, aspirin is prescribed for life and clopidogrel for 3 years. If a patient was taking an oral anticoagulant, clopidogrel monotherapy was prescribed in addition to the oral anticoagulant for 12 months or 3 years in the control and IVBT groups, respectively.
Study definitions and endpoints
Binary angiographic ISR was defined as >50% diameter narrowing within the stent or 5 mm of its edges. Target lesion revascularisation (TLR) was defined as revascularisation involving the stented segment or within 5 mm of the proximal or distal end of the stent, while target vessel revascularisation (TVR) was defined as any repeated revascularisation, either percutaneous or surgical, of any segment within the entire major coronary vessel in which the initial PCI was performed. Myocardial infarction (MI) was defined using the third universal definition. Adjudication of ST was done as per the Academic Research Consortium-2 criteria13. Major adverse cardiac events (MACE) were defined as a composite of all-cause death, MI, and TLR. A procedure was deemed angiographically successful if the final angiogram showed <30% residual stenosis with Thrombolysis in Myocardial Infarction flow grade 3 distally in the target vessel. The study’s primary endpoint was the incidence of MACE at 3-year follow-up. Secondary endpoints included the incidence of the individual components of MACE and ST.
Statistical analysis
Continuous variables are reported as mean±standard deviation or median (interquartile range [IQR]) and were compared using the Student’s t-test, the Mann-Whitney U test, or the Wilcoxon test, as appropriate. Categorical variables are reported as numbers (percentages) and were compared using the chi-square test or Fisher’s exact test, as appropriate. Propensity score (PS) stratification analysis was performed to account for baseline differences between patients assigned to the two treatment strategies. The PS was calculated using a multivariable logistic regression model with the dependent outcome as treatment with IVBT or a non-IVBT strategy. The PS model was generated in an iterative fashion using the method of Rosenbaum et al14. PS stratification was then used to analyse outcomes using cause-specific Cox proportional hazards regression models to account for the time-to-event nature of the data. The following variables were used for adjustment for PS stratification: age, sex, insulin-dependent diabetes, hypertension, hyperlipidaemia, dialysis, prior coronary artery bypass grafting (CABG), race (white vs non-white), current smoker, anaemia, peripheral arterial disease, left ventricular ejection fraction (LVEF) <40%, PCI presentation, B2/C lesion, bifurcation, calcification, and chronic total occlusion. The distribution of PS for the entire cohort and each treatment group was visually examined. Mutually exclusive strata were then generated based on the PS for the entire cohort, a process that was blinded to any outcome data to avoid bias in selection. The number of strata and their respective cutoff points were based on fulfilling previously established criteria and adequate balance in baseline covariates. The Cox proportional hazards model was used to calculate the unadjusted and adjusted hazard ratios (HRs). Estimated risks are expressed as unadjusted and adjusted HRs and 95% confidence intervals (CIs). Survival curves were generated using the Kaplan-Meier method and compared using the log-rank test. Clinical follow-up was censored at the date of death or the latest available follow-up. All reported p-values are 2-tailed, with p<0.05 considered significant. The events were set hierarchically based on their first occurrence. All statistical analyses used the SAS software, version 9.4 (SAS Institute) and Stata, version 18.0 (StataCorp). Dr Sharma had full access to all the data in the study and took responsibility for its integrity and data analysis.
Results
Baseline characteristics
Out of 25,641 procedures recorded in our PCI registry database between 1 January 2012 and 31 December 2019, 5,238 patients (20.4%) underwent PCI for DES-ISR. Among them, 647 patients (12.4%) had multilayer ISR: 453 patients (70%) were treated with IVBT, while the remaining 194 patients (30%) were treated with a non-IVBT PCI strategy (Figure 1).
Overall, baseline characteristics were similar between groups (Table 1). The mean age for the entire patient cohort was 66.6±9.9 years, and 25.5% were female. When compared with the no IVBT arm, patients treated with IVBT more commonly had prior CABG (44.6% vs 27.3%; p<0.001) and myocardial infarction (49.0% vs 45.4%; p=0.395) and less commonly had dialysis (3.5% vs 8.2%; p=0.011).
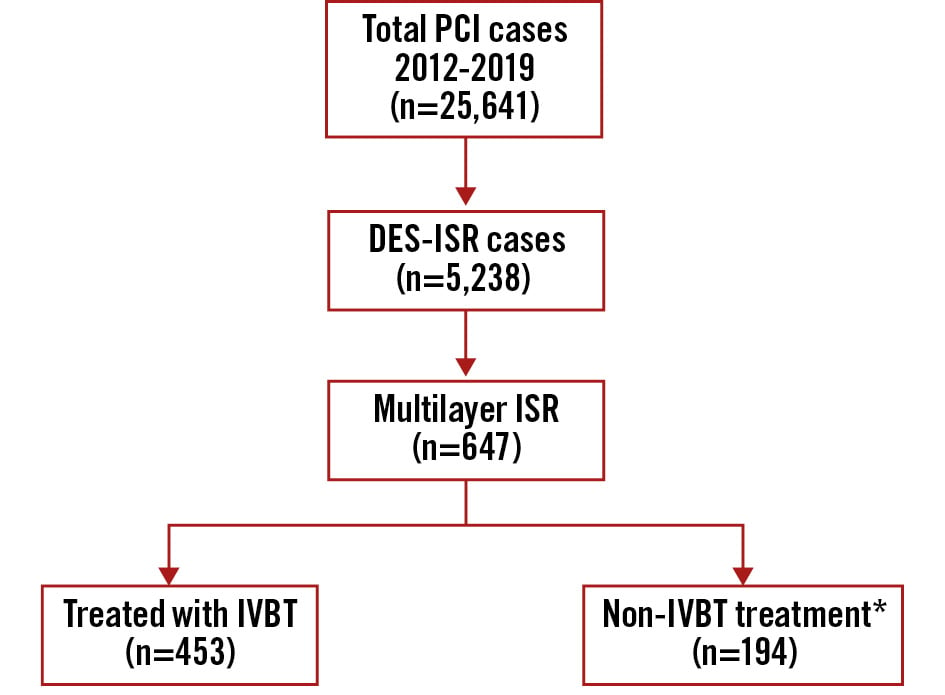
Figure 1. Graphical representation of the patients included in this analysis. *No drug-coated balloons used. DES: drug-eluting stent; ISR: in-stent restenosis; IVBT: intravascular brachytherapy; PCI: percutaneous coronary intervention
Table 1. Baseline clinical characteristics.
| Characteristics | Overall N=647 |
IVBT N=453 (70.0%) |
No IVBT N=194 (30.0%) |
p-value |
|---|---|---|---|---|
| Age, years | 66.6±9.9 | 66.3±9.6 | 67.2±10.7 | 0.312 |
| BMI, kg/m2 | 28.9±5.6 | 29.3±5.6 | 28.1±5.5 | 0.013 |
| Female sex | 165 (25.5) | 115 (25.4) | 50 (25.9) | 0.890 |
| Race/ethnicity | <0.001 | |||
| Caucasian | 342 (56.2) | 259 (60.1) | 83 (46.6) | |
| African American | 51 (8.4) | 26 (6.0) | 25 (14.0) | |
| Asian | 113 (18.6) | 79 (18.3) | 34 (19.1) | |
| Hispanic | 82 (13.5) | 50 (11.6) | 32 (18.0) | |
| Others | 21 (3.4) | 17 (3.9) | 4 (2.2) | |
| Medical history | ||||
| Current smoker | 80 (12.4) | 46 (10.2) | 34 (17.5) | 0.009 |
| Family history of CAD | 166 (25.7) | 121 (26.7) | 45 (23.2) | 0.348 |
| Anaemia | 332 (52.3) | 240 (53.5) | 92 (49.5) | 0.360 |
| Diabetes mellitus | 379 (58.6) | 272 (60.0) | 107 (55.2) | 0.247 |
| Insulin-dependent | 196 (30.3) | 129 (28.5) | 67 (34.5) | 0.124 |
| Hypertension | 636 (98.5) | 449 (99.3) | 187 (96.4) | 0.010 |
| Hyperlipidaemia | 636 (98.3) | 447 (98.7) | 189 (97.4) | 0.319 |
| Lung disease | 48 (7.4) | 31 (6.8) | 17 (8.8) | 0.393 |
| Peripheral artery disease | 101 (15.6) | 73 (16.1) | 28 (14.4) | 0.589 |
| Cerebrovascular disease | 99 (15.3) | 69 (15.2) | 30 (15.5) | 0.940 |
| Atrial fibrillation | 70 (10.8) | 45 (9.9) | 25 (12.9) | 0.268 |
| Dialysis | 32 (4.9) | 16 (3.5) | 16 (8.2) | 0.011 |
| Chronic kidney disease | 202 (31.2) | 139 (30.7) | 63 (32.5) | 0.653 |
| Prior MI | 310 (47.9) | 222 (49.0) | 88 (45.4) | 0.395 |
| Prior CABG | 255 (39.4) | 202 (44.6) | 53 (27.3) | <0.001 |
| LVEF, % | 52.7±11.2 | 53.2±10.8 | 51.6±12.3 | 0.135 |
| PCI presentation | ||||
| Asymptomatic | 56 (8.7) | 53 (11.8) | 3 (1.6) | <0.001 |
| Stable angina | 322 (50.2) | 229 (50.8) | 93 (48.7) | 0.629 |
| Unstable angina | 203 (31.6) | 158 (35.0) | 45 (23.6) | 0.004 |
| NSTEMI | 60 (9.3) | 11 (2.4) | 49 (25.7) | <0.001 |
| STEMI | 1 (0.2) | 0 (0) | 1 (0.5) | 0.298 |
| Data are presented as mean±standard deviation or n (%). BMI: body mass index; CABG: coronary artery bypass grafting; CAD: coronary artery disease; IVBT: intravascular brachytherapy; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction | ||||
Procedural characteristics and outcomes
Femoral artery access was preferred in the majority of cases in both groups, especially among patients in the no IVBT group (76.8% vs 66.9%; p=0.012) (Table 2). A similar proportion of patients had multivessel disease (64.5% vs 68.6%; p=0.314), although bifurcation lesions were less common in the IVBT group (8.6% vs 16.5%; p=0.003). Rotational atherectomy was the dominant form of atherectomy and was more frequently used in the IVBT group (31.1% vs 19.7%; p=0.003). Overall use of intravascular imaging was low (<10%), and intravascular ultrasound (IVUS) was the preferred modality across the groups. Those treated with IVBT were less likely to undergo IVUS-guided coronary intervention (6.0% vs 12.9%; p=0.003). DES implantation was significantly more common in the non-IVBT strategy group (n=50, 25.8%) compared to the IVBT-treated group (n=2, 0.4%). All patients achieved immediate angiographic success, and the incidence of procedural complications was low and similar across the groups. All patients were alive at discharge. On discharge, oral anticoagulation was prescribed in 10% of all cases, with no significant difference between the two study groups. The remaining patients were discharged on DAPT.
Table 2. Baseline procedural characteristics.
| Characteristics | Overall N=647 |
IVBT N=453 (70.0%) |
No IVBT N=194 (30.0%) |
p-value |
|---|---|---|---|---|
| Access | ||||
| Radial access | 187 (28.9) | 144 (31.8) | 43 (22.2) | 0.013 |
| Femoral access | 452 (69.9) | 303 (66.9) | 149 (76.8) | 0.012 |
| PCI vessels | ||||
| Left main | 47 (7.3) | 21 (4.6) | 26 (13.4) | <0.001 |
| LAD | 348 (53.9) | 213 (47.1) | 135 (69.6) | <0.001 |
| LCx | 338 (52.3) | 215 (47.6) | 123 (63.4) | <0.001 |
| RCA | 285 (44.1) | 191 (42.3) | 94 (48.5) | 0.146 |
| Bypass graft intervention | 49 (7.6) | 49 (10.8) | 0 (0) | <0.001 |
| Lesions and stents | ||||
| B2/C lesion | 447 (69.1) | 343 (75.7) | 104 (53.6) | <0.001 |
| Calcification (mod/severe) | 51 (7.9) | 33 (7.3) | 18 (9.3) | 0.389 |
| Bifurcation | 71 (11.0) | 39 (8.6) | 32 (16.5) | 0.003 |
| Chronic total occlusion | 42 (6.5) | 24 (5.3) | 18 (9.3) | 0.060 |
| Total stent length, mm | 52.7±42.8 | 44.0±39.0 | 61.2±45.0 | 0.044 |
| No. of lesions treated | 1.9±1.1 | 1.7±1.0 | 2.3±1.3 | <0.001 |
| Max stent diameter, mm | 3.3±0.4 | 3.3±0.3 | 3.3±0.5 | 0.968 |
| SYNTAX score | 12.7±11.0 | 11.9±10.7 | 14.2±11.3 | 0.028 |
| Use of imaging and devices | ||||
| IVUS | 52 (8.0) | 27 (6.0) | 25 (12.9) | 0.003 |
| OCT | 12 (1.9) | 9 (2.0) | 3 (1.5) | 1.000 |
| Atherectomy | ||||
| Orbital atherectomy | 21 (3.2) | 18 (4.0) | 3 (1.5) | 0.110 |
| Rotational atherectomy | 179 (27.7) | 141 (31.1) | 38 (19.7) | 0.003 |
| Excimer laser | 7 (1.1) | 2 (0.4) | 5 (2.6) | 0.028 |
| Drug-eluting stent placement | 52 (8.0) | 2 (0.4) | 50 (25.8) | <0.001 |
| Procedural complications | ||||
| Dissection | 2 (0.3) | 1 (0.2) | 1 (0.5) | 0.510 |
| Side branch closure | 3 (0.5) | 3 (0.7) | 0 (0.0) | 1.000 |
| Perforation | 4 (0.6) | 3 (0.7) | 1 (0.5) | 1.000 |
| Tamponade | 1 (0.2) | 0 (0) | 1 (0.5) | 0.299 |
| Slow flow/no flow | 11 (1.7) | 7 (1.5) | 4 (2.1) | 0.741 |
| Vessel closure | 1 (0.2) | 0 (0) | 1 (0.7) | 0.228 |
| Periprocedural MI | 27 (4.2) | 20 (4.4) | 7 (3.6) | 0.215 |
| Periprocedural bleeding | 12 (1.9) | 6 (1.3) | 6 (3.1) | 0.199 |
| Discharge medication | ||||
| DAPT | 591 (91.6) | 416 (92.0) | 175 (90.7) | 0.567 |
| Aspirin | 591 (91.6) | 416 (92.0) | 175 (90.7) | 0.567 |
| P2Y12 inhibitor | 643 (99.7) | 452 (100) | 191 (99.0) | 0.089 |
| Oral anticoagulant | 66 (10.2) | 47 (10.4) | 19 (9.9) | 0.848 |
| Statin | 607 (94.1) | 426 (94.2) | 181 (93.8) | 0.818 |
| Beta blocker | 541 (83.9) | 385 (85.2) | 156 (80.8) | 0.169 |
| Data are presented as n (%) or mean±standard deviation. DAPT: dual antiplatelet therapy; IVBT: intravascular brachytherapy; IVUS: intravascular ultrasound; LAD: left anterior descending artery; LCx: left circumflex artery; MI: myocardial infarction; OCT: optical coherence tomography; PCI: percutaneous coronary intervention; RCA: right coronary artery | ||||
Clinical outcomes
For the overall study population, the median duration of follow-up was 1,096 (IQR 506-1,096) days. The incidence of the primary endpoint, MACE, at 3-year follow-up, was lower in the IVBT-treated group compared to the non-IVBT-treated group (PS-adjusted analysis: 39.5% and 47.8%; HR 0.73, 95% CI: 0.53-0.99; p=0.044) (Table 3, Central illustration). TLR was numerically lower in the IVBT-treated group (27.6% vs 34.5%; HR 0.73, 95% CI: 0.50-1.07; p=0.111). The incidences of all-cause death (12.3% vs 15.3%; HR 0.70, 95% CI: 0.40-1.23; p=0.215) and MI after discharge (5.4% vs 11.5%; HR 0.68, 95% CI: 0.33-1.38; p=0.283) were comparable between the IVBT and no IVBT groups, respectively. At 1-year follow-up, there was no significant difference in the rate of stent thrombosis between patients treated with IVBT (n=3, 0.67%) and those treated with a non-IVBT strategy (n=4, 2.0%); p=0.116. Kaplan-Meier survival analysis revealed a lower risk of 3-year MACE in patients treated with an IVBT compared to a non-IVBT strategy (log-rank p=0.017) (Central illustration B). A landmark analysis at 1-year follow-up showed that the lower incidence of MACE was maintained after 1 year of follow-up (Figure 2).
For patients treated with IVBT, the association between the use of atherectomy and clinical outcomes at 3 years was examined (Table 4). On crude (HR 1.03, 95% CI: 0.73-1.47; p=0.849) and adjusted analysis (HR 1.01, 95% CI: 0.70-1.45; p=0.975), the incidence of the primary outcome, MACE, was similar between patients who had lesion preparation with atherectomy compared to those who had IVBT but no atherectomy. There were no significant differences in the individual components of MACE between patients treated with IVBT with or without adjunctive atherectomy.
Table 3. PS-stratified association between IVBT and outcomes 3 years after PCI.
| IVBT N=453 (70.0%) |
No IVBT N=194 (30.0%) |
PS-stratifiedhazard ratio (95% CI)* | p-value | |
|---|---|---|---|---|
| All-cause death | 45 (12.3) | 23 (15.3) | 0.70 (0.40-1.23) | 0.215 |
| MI | 20 (5.4) | 17 (11.5) | 0.68 (0.33-1.38) | 0.283 |
| TVR | 118 (33.8) | 56 (39.0) | 0.82 (0.58-1.16) | 0.257 |
| TLR | 95 (27.6) | 50 (34.5) | 0.73 (0.50-1.07) | 0.111 |
| Stroke | 2 (0.7) | 0 (0) | N/A | N/A |
| Death or MI | 58 (15.6) | 38 (23.4) | 0.67 (0.42-1.05) | 0.079 |
| MACE | 141 (39.5) | 77 (47.8) | 0.73 (0.53-0.99) | 0.044 |
| Data are presented as n (%). *PS-stratified outcomes according to age, sex, diabetes with insulin, hypertension, hyperlipidaemia, dialysis, prior CABG, race (white vs non-white), current smoker, anaemia, PAD, LVEF <40%, PCI presentation, B2/C lesion, bifurcation, calcification (moderate/severe), and chronic total occlusion. The percentages mentioned above represent Kaplan-Meier rates 3 years after the procedure. Missing PS values were imputed using linear regression. CABG: coronary artery bypass grafting; CI: confidence interval; IVBT: intravascular brachytherapy; LVEF: left ventricular ejection fraction; MACE: major adverse cardiac events; MI: myocardial infarction; N/A: not applicable; PAD: peripheral arterial disease; PCI: percutaneous coronary intervention; PS: propensity score; TLR: target lesion revascularisation; TVR: target vessel revascularisation | ||||
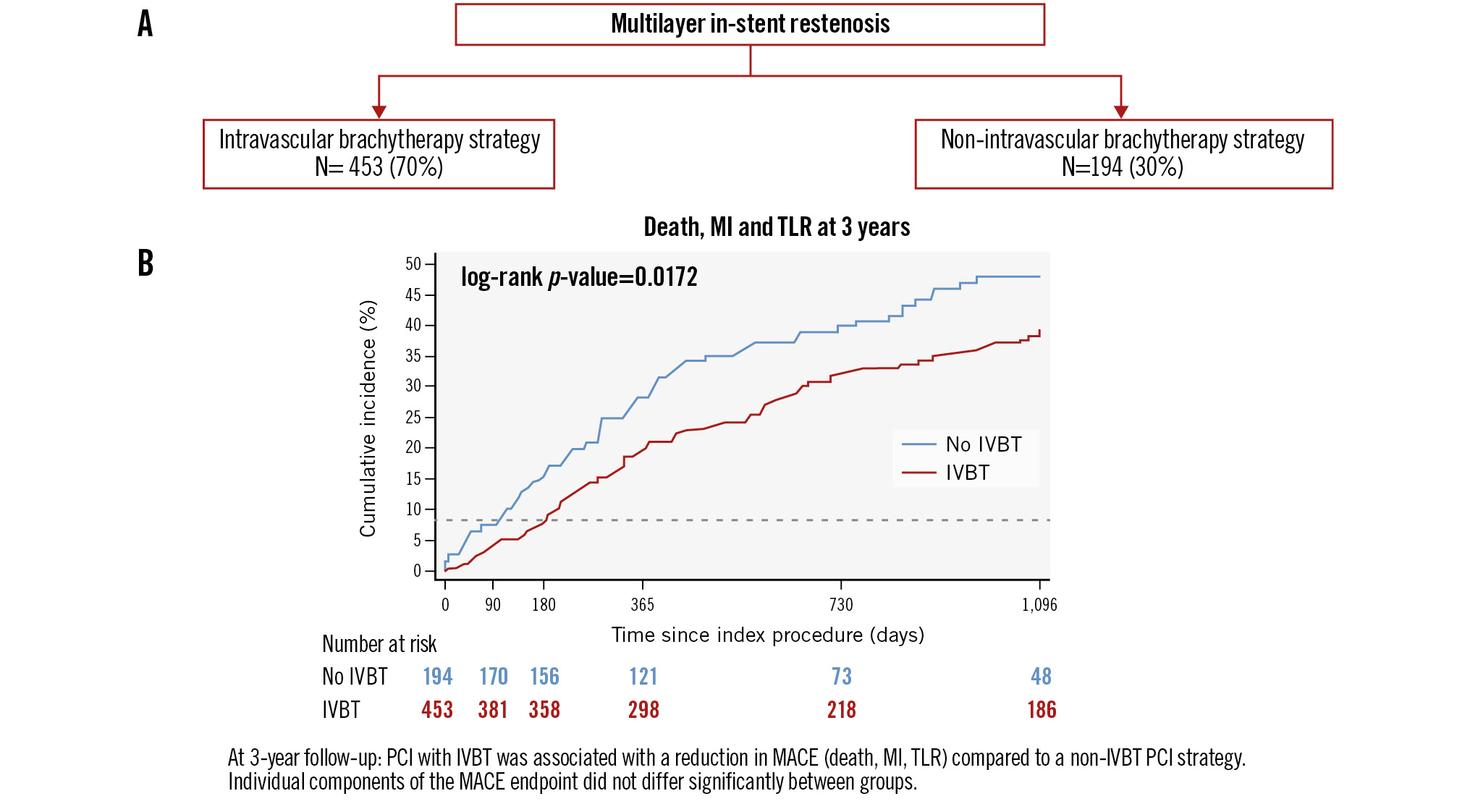
Central illustration. Clinical outcomes at 3 years after treatment of multilayer in-stent restenosis with an IVBT or a non-IVBT strategy. A) Patients treated for multilayer ISR (n=647) and PCI strategies; (B) Kaplan-Meier estimates for MACE at 3-year follow-up (IVBT vs non-IVBT strategy). IVBT: intravascular brachytherapy; MACE: major adverse cardiac events; MI: myocardial infarction; PCI: percutaneous coronary intervention; TLR: target lesion revascularisation
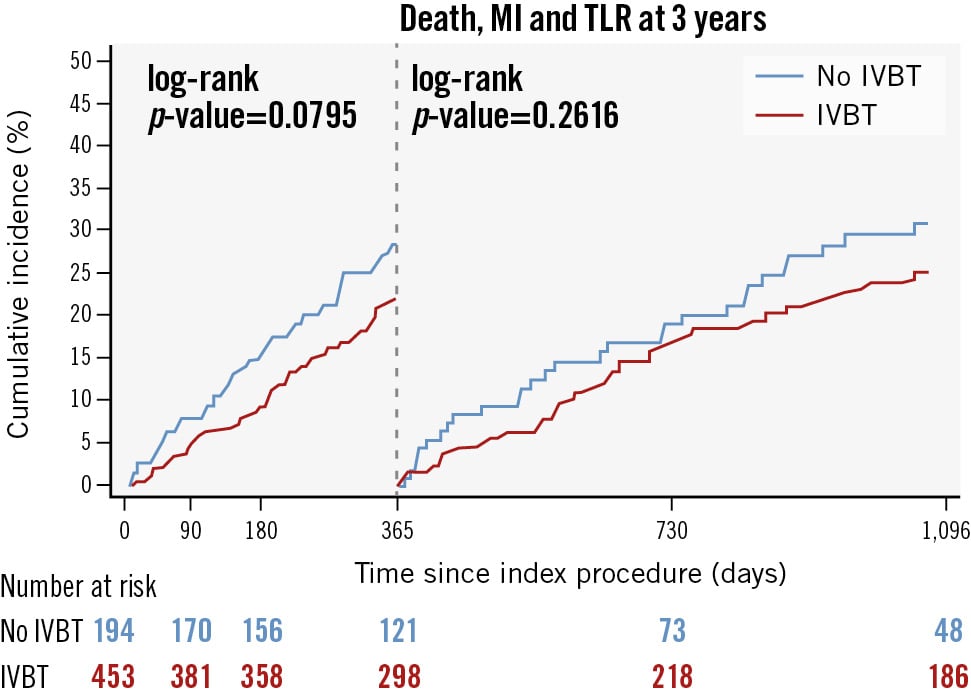
Figure 2. Kaplan-Meier estimates for MACE at 1 year as a landmark time and 3 years after PCI. IVBT: intravascular brachytherapy; MACE: major adverse cardiac events; MI: myocardial infarction; PCI: percutaneous coronary intervention; TLR: target lesion revascularisation
Table 4. Association between atherectomy and clinical outcomes 3 years after IVBT treatment.
| Atherectomy N=159 (35.2%) |
No atherectomy N=293 (64.8%) |
Hazard ratio (95% CI) | p-value | Adjusted hazard ratio (95% CI)* | p-value | |
|---|---|---|---|---|---|---|
| All-cause death | 14 (11.7) | 31 (12.5) | 0.95 (0.51-1.79) | 0.886 | 1.13 (0.58-2.22) | 0.723 |
| MI | 7 (6.3) | 13 (5.0) | 1.12 (0.45-2.80) | 0.816 | 1.03 (0.39-2.68) | 0.957 |
| TVR | 39 (34.4) | 79 (33.5) | 1.03 (0.70-1.51) | 0.881 | 1.01 (0.67-1.50) | 0.978 |
| TLR | 31 (28.0) | 64 (27.4) | 1.00 (0.65-1.54) | 0.988 | 0.91 (0.58-1.44) | 0.683 |
| Stroke | 0 (0) | 2 (1.0) | N/A | N/A | N/A | N/A |
| Death or MI | 19 (15.8) | 39 (15.5) | 1.02 (0.59-1.77) | 0.943 | 1.07 (0.60-1.91) | 0.822 |
| Death, MI, or TVR | 53 (43.4) | 105 (42.0) | 1.04 (0.75-1.45) | 0.800 | 1.04 (0.74-1.47) | 0.832 |
| Death, MI, or TLR | 47 (40.3) | 94 (39.2) | 1.03 (0.73-1.47) | 0.849 | 1.01 (0.70-1.45) | 0.975 |
| Data are presented as n (%). *Outcomes are adjusted for age, sex, diabetes with insulin, hypertension, hyperlipidaemia, dialysis, prior CABG, race (white vs non-white), current smoker, PCI presentation, B2/C lesion, bifurcation. The percentages mentioned above represent Kaplan-Meier rates 3 years after the procedure. CI: confidence interval; IVBT: intravascular brachytherapy; MI: myocardial infarction; N/A: not applicable; TLR: target lesion revascularisation; TVR: target vessel revascularisation | ||||||
Discussion
The present study represents the largest analysis of long-term outcomes after IVBT for multilayer DES-ISR (≥2 stents). The main findings of our study, with a 3-year follow-up, are the following: (1) Patients treated with an IVBT PCI strategy experienced a lower risk of MACE (all-cause death, TLR, MI) compared to those treated with a non-IVBT PCI strategy; (2) TLR is frequent (about 1 in 3 cases) among patients treated for multilayer ISR and continues to accrue throughout 3-year follow-up regardless of the treatment modality; (3) on landmark analysis at 1-year follow-up, no “catch-up phenomenon” was seen in patients treated with IVBT.
Despite a significant reduction in the incidence of ISR with DES compared to BMS, ISR remains an Achilles’ heel of coronary stents3415. Even more so, a higher rate of ISR recurrence can be expected after multilayer stenting, as each additional stent layer reduces the minimal stent area, a known predictor of future ISR. Furthermore, correcting index stent underexpansion can be near impossible when multiple stent layers are present. IVBT was initially introduced for treating BMS-ISR; hence, the evidence underpinning IVBT for ISR is mainly based on patients with BMS rather than contemporary DES. Furthermore, when DES became commercially available, the use of IVBT for treating BMS-ISR was significantly reduced16. While DES and drug-coated balloons (DCBs) remain the recommended treatment for treating ISR, there has been a resurgence in IVBT use across the USA. This has been driven by the limited armamentarium for treating multilayer ISR and the appealing nature of a “metal-free” strategy that can simplify complex lesions (e.g., bifurcation lesions) and avoid lumen area loss.
The extensive vascular disease profile of patients presenting with multilayer ISR is highlighted in this study. Despite a mean age of 66.6±9.9 years, almost one-half of patients in the IVBT arm had prior CABG, and more than half of the patients had diabetes mellitus across both groups. This is in keeping with previous studies on multilayer ISR and highlights that surgical treatment of multilayer ISR in patients with a history of prior CABG is often an unattractive or unfeasible option1117. Furthermore, ISR is not a benign condition, with up to 50% of ISR patients presenting with unstable angina and 10-20% having an acute MI4111718. These findings are also mirrored in our study. Acute coronary syndrome is infrequently treated with IVBT, given the increased risk of worsening thrombus and the association of IVBT with more frequent TLR when used in this clinical setting317.
This study provides reassuring data on the safety of IVBT for treating multilayer ISR, with comparable periprocedural complication rates to those treated with a non-IVBT strategy. One year after multilayer ISR IVBT, 10-12% of patients will require TLR121819. In our patient cohort, the rate of TLR increased to 27.6% at 3-year follow-up, with a numerically lower incidence compared to those treated with a non-IVBT PCI strategy. This finding aligns with the study by Megaly et al (TLR 29.4%, n=42) but is higher than that seen by Negi et al (TLR 19.4%, n=30) and lower than that recently reported by Ho et al (TLR 45.8%)11171820. The higher incidence of TLR in the latter study may be explained by the fact that 20.9% of patients presented with an acute MI and 62% had a prior CABG, both of which have recently been shown to be independent predictors of TVR during follow-up21. Moreover, in the study by Negi et al, intravascular imaging, known to improve interventional outcomes, was used in 96% of cases, and no underexpansion was recorded, likely contributing to the lower TLR rate. The increasing rate of TLR over time in each of these studies may be attributed to a “catch-up" phenomenon. This phenomenon was described in Scripps Coronary Radiation to Inhibit Proliferation Post Stenting (SCRIPPS) and Washington Radiation for In-Stent restenosis Trial (WRIST), where the effect of IVBT on inhibiting ISR recurrence weakened after 6 months, and TLR rates increased in contrast to our study2223. The absence of a “catch-up” phenomenon in our IVBT patient cohort may be explained by treatment of DES-ISR rather than BMS-ISR, greater lesion modification and debulking, avoidance of re-stenting in the IVBT arm, and prolonged DAPT for at least 3 years. Moreover, the results of this analysis exhibit enhanced robustness and innovation compared to previous investigations due to the larger cohort size and the inclusion of a comparator group.
IVBT extends clinicians’ armamentarium in clinical practice for treating recurrent ISR. However, challenges exist, as IVBT is only available in a minority of catheterisation laboratories, and treatment requires a multidisciplinary approach (radiation oncology specialists and physicists)4. Some of these limitations may be overcome by staging procedures that require IVBT (e.g., lesion preparation on index visit and return visit for IVBT) to facilitate the coordination of a multidisciplinary team and referral of recurrent ISR cases to specialist IVBT centres. In addition, uncertainty remains over the optimal duration of DAPT after IVBT, given the association of brachytherapy with thrombosis due to delayed endothelisation924. Although not guideline based, at least 12 months of DAPT is generally recommended based on a comparison of the WRIST 12 and WRIST PLUS studies, which found significantly lower rates of MACE and TLR in those who received at least 12 months of DAPT, even though stent thrombosis rates were similar25. Furthermore, animal studies have shown incomplete vascular healing 6 months after IVBT26. The uncertainty over the optimal duration of DAPT is reflected in registry studies, showing DAPT prescribed for anywhere between 1 year and lifelong91719.
The U.S. Food and Drug Administration recently approved the AGENT DCB (Boston Scientific) for treating ISR in the USA. During the study period, DCBs were unavailable in the United States. DCBs have the advantage of being readily available on the shelf, having fewer logistical issues, and necessitating a shorter duration of DAPT27. Furthermore, DCBs are widely used across Europe and recommended by the European Society of Cardiology for treating ISR28. Nonetheless, multilayer ISR may remain a niche indication for IVBT. Studies analysing DCBs for treating ISR have included a minority of patients with multilayer ISR (≥2 layers). A Tokyo registry study showed that DCBs were less effective when there were >2 layers of the stent (41% vs 15% TLR at 1 year)2930. Hence, further research is needed to assess the efficacy of DCBs in multilayer ISR. The present study can be a benchmark for comparing DCB and IVBT efficacy in treating multilayer ISR. However, a dedicated randomised controlled trial of the two therapies would ideally be undertaken to help define the optimal treatment of multilayer ISR.
Limitations
Certain limitations of our study warrant attention. First, although the data included in this study are extracted from an extensive database of over 25,000 patients, the results represent the outcomes at a single centre, which limits the study’s external validity. Moreover, the frequency of ISR PCI was 20.4% (national average ≈10%), reflecting the tertiary referral nature of the hospital and the known access to IVBT. Second, although we employed a meticulous statistical methodology to mitigate the influence of confounding variables on the outcomes reported, it remains impossible to eradicate the risk of unmeasured confounders. Third, in keeping with previous studies from our institution, we did not adjudicate cardiovascular death, given the fundamental challenge of accurately recording this variable, and hence reported all-cause death. Fourth, although IVBT is usually the preferred treatment modality for multilayer ISR at this institution, the treatment decision remains ultimately at the treating physician’s discretion. Fifth, in keeping with the national frequency of intravascular imaging use during the study period, a minority of patients in this study underwent intravascular imaging. Sixth, our database does not record information on medication compliance or lifestyle modification (e.g., smoking cessation) beyond discharge, which are known factors impacting the incidence of MACE during follow-up. Seventh, no data were available on the rate of ST beyond 1 year or following DAPT cessation in either group. Moreover, the difference in antiplatelet regimens prescribed after PCI in each group represents a fundamental difference in how patients were medically managed after their procedure. Eighth, although this study represents the largest investigation of long-term outcomes for ISR treated with IVBT, the sample size remains relatively small for adjusting for the many potential confounders, as detailed in Supplementary Table 1. Future research, such as a multicentre, patient-level, pooled-data analysis, could provide more substantial and impactful insights into the treatment of ISR with IVBT. Finally, it was not possible to accurately record the incidence of bleeding events beyond 1 year post-procedure.
Conclusions
In this analysis of prospectively collected data, patients with multilayer DES-ISR treated with an IVBT PCI strategy had a lower risk of MACE at 3-year follow-up than those treated with a non-IVBT strategy. The high incidence of TLR at 3-year follow-up in both groups underlines the need for further research on multilayer ISR treatment. Prospective studies comparing IVBT and DCBs are now required to define the optimal treatment of multilayer ISR.
Impact on daily practice
Multilayer (≥2 layers) in-stent restenosis (ISR) represents a clinical challenge for physicians, with limited treatment options and a high incidence of ISR recurrence. The results of this study describe the safety of intravascular brachytherapy (IVBT) for treating multilayer drug-eluting stent ISR and a reduced rate of major adverse cardiac events (MACE) at 3-year follow-up when IVBT is used over a non-IBVT treatment strategy. Furthermore, this study underlines the unmet clinical need in treating multilayer ISR, which has a high rate of MACE irrespective of the treatment modality used.
Acknowledgements
We gratefully acknowledge the support and expertise of the Mount Sinai Fuster Heart Hospital research team.
Conflict of interest statement
R. Mehran has received institutional research grants from Abbott, Abiomed, Applied Therapeutics, Arena, AstraZeneca, Bayer, Biosensors, Boston Scientific, Bristol-Myers Squibb, Cardiawave, CellAegis, CERC, Chiesi, Concept Medical, CSL Behring, DSI, Insel Gruppe, Medtronic, Novartis Pharmaceuticals, OrbusNeich, Philips, Transverse Medical, and Zoll; has received personal fees from the American College of Cardiology, Boston Scientific, the California Institute for Regenerative Medicine, Cine-Med Research, Janssen, WebMD, and the Society for Cardiovascular Angiography and Interventions; has received consulting fees paid to the institution from Abbott, Abiomed, AM-Pharma, Alleviant Medical, Bayer, Beth Israel Deaconess, Cardiawave, CeloNova, Chiesi, Concept Medical, DSI, Duke University, Idorsia Pharmaceuticals, Medtronic, Novartis, and Philips; holds equity (<1%) in Applied Therapeutics, Elixir Medical, STEL, and ControlRad (spouse); has served as a scientific advisory board member for the American Medical Association; and has a spouse who has served as a scientific advisory board member for Biosensors. The other authors have no conflicts of interest relevant to the contents of this paper to declare.
Supplementary data
To read the full content of this article, please download the PDF.

