Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Transcatheter edge-to-edge repair (TEER) is among the treatments for functional mitral regurgitation (FMR), but its benefits over guideline-directed medical therapy (GDMT) alone are discordant. We conducted a meta-analysis of randomised trials comparing long-term outcomes between these treatment strategies.
Aims: We aimed to compare long-term clinical outcomes between TEER plus GDMT and GDMT alone in symptomatic moderate-to-severe FMR.
Methods: Major electronic databases were searched for randomised trials comparing TEER plus GDMT with GDMT alone in FMR. The primary outcome was death or first hospitalisation due to heart failure at 24 months. The key secondary outcome was first hospitalisation due to heart failure at 24 months. Summary hazard ratios (HRs) with 95% confidence intervals (CIs) were computed by mixed-effects Cox models based on reconstructed time-to-first event individual patient data and random-effects models based on study-level data.
Results: Three randomised trials (MITRA-FR, COAPT, and RESHAPE-HF2) were included, for a total of 1,422 patients assigned to TEER plus GDMT (n=703) or GDMT alone (n=719). The primary outcome was significantly lower in the TEER plus GDMT group compared with the GDMT-alone group by one-stage analysis (HR 0.72, 95% CI: 0.56-0.92; p=0.010). However, the two-stage analysis marginally failed to confirm this result (HR 0.72, 95% CI: 0.51-1.00; p=0.052) and showed substantial heterogeneity (I²=80.3%; p=0.006). Hospitalisation due to heart failure was significantly lower in the TEER plus GDMT group, regardless of the statistical method used (one-stage: HR 0.65, 95% CI: 0.48-0.88; p=0.006; two-stage: HR 0.66, 95% CI: 0.45-0.96; p=0.031). However, heterogeneity was substantial (I²=81.2%; p=0.005). All-cause death and cardiovascular death at 24 months were not significantly different between treatment groups but became significant after excluding MITRA-FR in the leave-one-out analysis.
Conclusions: In symptomatic moderate-to-severe FMR, TEER plus GDMT significantly reduces death or hospitalisation due to heart failure and hospitalisation due to heart failure at 24 months.
Results
The search and selection of trials are shown in Supplementary Figure 1 and Supplementary Table 2. A total of three randomised controlled trials (MITRA-FR, COAPT, and RESHAPE-HF2)61112131415 (Supplementary Appendix 1) were included in the meta-analysis, encompassing a combined population of 1,422 patients with symptomatic FMR, of whom 703 were assigned to TEER plus GDMT and 719 to GDMT alone. The main characteristics of the trials are presented in Table 1, Table 2, Supplementary Table 3, and Supplementary Table 4. Some characteristics, primarily sex, left ventricular end-diastolic volume, FMR severity, atrial fibrillation, and GDMT showed heterogeneity across trials. Specifically, patients in COAPT and in RESHAPE-HF2 presented with smaller left ventricular end-diastolic volumes (194±69 mL vs 191±73 mL in COAPT; 200±24 mL vs 206±23 mL in RESHAPE-HF2) than those in MITRA-FR (255.6±63 mL vs 258.8±71 mL). The ischaemic aetiology of FMR was predominant, identified in almost two-thirds of patients in each trial, with high prevalences of prior revascularisation and coronary artery bypass grafting. The effective regurgitant orifice area was largest in COAPT (0.41 cm²), followed by MITRA-FR (0.31 cm²) and RESHAPE-HF2 (0.23 cm²). Atrial fibrillation was less prevalent in MITRA-FR (33.6%) compared with COAPT (55.2%) and RESHAPE-HF2 (48.1%). New York Heart Association (NYHA) Class III-IV was the most frequent, ranging from 63.1-71.1% in the MITRA-FR trial to 76.4-74.1% in the RESHAPE-HF2 trial. Loop diuretic use was prescribed to almost all patients, ranging from 89.1% in COAPT to 98.7% in MITRA-FR. COAPT had a lower proportion of patients on angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or angiotensin receptor-neprilysin inhibitors (67.3%) compared with MITRA-FR (84.8%) and RESHAPE-HF2 (87.9%). Consistently, beta blockers and mineralocorticoid receptor antagonists were more prevalent in RESHAPE-HF2 (95.8% and 82.2%, respectively) compared with MITRA-FR (89.8% and 54.8%, respectively) and COAPT (90.4% and 50.2%, respectively). Sodium-glucose co-transporter 2 inhibitor use was reported only in RESHAPE-HF2, as it entered clinical practice for heart failure treatment after the other two trials had been completed. The quality of trials was high overall and, except for a possible bias due to the unfeasibility of masking, there were no significant concerns (Supplementary Figure 2).
Table 1. Design of the included trials.
| MITRA-FR (2019)1113 | COAPT (2019)1214 | RESHAPE-HF2 (2024)615 | |
|---|---|---|---|
| Sample size | 304 | 614 | 505 |
| (TEER vs GDMT) | (152 vs 152) | (302 vs 312) | (250 vs 255) |
| Study population | Heart failure and FMR | Heart failure and FMR | Heart failure and FMR |
| Accrual period, years | 3.3 | 4.5 | 8.7 |
| Centres | 37 | 78 | 30 |
| Patients/site | 8.2 | 7.8 | 16.8 |
| Patients/site/year | 2.5 | 1.8 | 1.9 |
| Countries | France | United States | Czech Republic, Denmark, Germany, Greece, Italy, Poland, Portugal, Spain, United Kingdom |
| Clinical inclusion criteria | NYHA II-IV Not a candidate for surgery ≥1 hospitalisation(s) due to heart failure <12 months | NYHA II-IVa ≥1 hospitalisation(s) due to heart failure <12 months or BNP ≥300 pg/mL or NT-proBNP ≥1,000 pg/mL after GDMT Not a candidate for surgery | NYHA II-IV ≥1 hospitalisation(s) due to heart failure <12 months or BNP ≥300 pg/mL or NT-proBNP ≥1,000 pg/mL after GDMT Surgery is not preferable |
| Echocardiographic inclusion criteria | Grade 3+ or 4+ FMR EROA >20 mm2 and/or RV >30 mL/beat LVEF 15-40% | Grade 3+ or 4+ FMR LVEDD ≤70 mm LVEF 20-50% | Grade 3+ or 4+ FMR LVEF 15-35% and NYHA II or LVEF ≤45% and NYHA III-IV |
| GDMT | At the investigator’s discretion | Stable maximal doses of GDMT and cardiac resynchronisation therapy | GDMT with no dose changes except diuretics for ≤2 weeks |
| TEER system | MitraClip | MitraClip | MitraClip |
| Hypothesis | Superiority | Superiority | Superiority |
| Primary outcomes | Death or hospitalisation due to heart failure at 12 months | Hospitalisation due to heart failure at 24 months Device-related complications at 12 months | Cardiovascular death or hospitalisation due to heart failure at 24 months Hospitalisation due to heart failure at 24 months Change in KCCQ at 12 months |
| Key secondary outcomes | All-cause death, hospitalisation due to heart failure, and cardiovascular death at 12 months | All-cause death or hospitalisation due to heart failure, and cardiovascular death at 24 months | All-cause death, hospitalisation due to heart failure, and cardiovascular death at 24 months |
| Maximum available follow-up | 24 months | 60 months | 24 months |
| BNP: B-type natriuretic peptide; EROA: effective regurgitant orifice area; FMR: functional mitral regurgitation; GDMT: guideline-directed medical therapy; KCCQ: Kansas City Cardiomyopathy Questionnaire; LVEDD: left ventricular end-diastolic dimension; LVEF: left ventricular ejection fraction; NT-proBNP: N-terminal pro-B-type natriuretic peptide; NYHA: New York Heart Association; RV: regurgitant volume; TEER: transcatheter edge-to-edge repair | |||
Table 2. Baseline characteristics across trials.
| Baseline characteristics | MITRA-FR (2019)11 | COAPT (2019)12 | RESHAPE-HF2 (2024)6 | |||
|---|---|---|---|---|---|---|
| TEER (152) | GDMT (152) | TEER (302) | GDMT (312) | TEER (250) | GDMT (255) | |
| Age, years | 70.1±10.1 | 70.6±9.9 | 71.7±11.8 | 72.8±10.5 | 70.0±10.4 | 69.4±10.7 |
| Female | 32 (21.1) | 45 (19.6) | 101 (33.3) | 120 (38.5) | 55 (22.0) | 44 (17.2) |
| NYHA III-IV | 96 (63.1) | 108 (71.1) | 172 (57.0) | 201 (64.6) | 191 (76.4) | 189 (74.1) |
| NT-proBNP, pg/mL | 3,407 (1,948-6,790) | 3,292 (1,937-6,343) | 5,174.3±6,566.6 | 5,943.9±8,437.6 | 2,651 (1,630-4,918) | 2,816 (1,306-5,496) |
| Ischaemic cardiomyopathy | 95 (62.5) | 85 (56.3) | 184 (60.9) | 189 (60.6) | 162 (64.8) | 167 (65.4) |
| eGFR, mL/min/1.73m² | 48.8±19.7 | 50.2±20.1 | 50.9±28.5 | 47.8±25.0 | 54.9±19.0 | 56.7±23.3 |
| Atrial fibrillation | 49 (34.5) | 48 (32.7) | 173 (57.3) | 166 (53.2) | 118 (47.2) | 125 (49.0) |
| EuroSCORE II | 6.6 (3.5-11.9) | 5.9 (3.4-10.4) | – | – | – | – |
| STS score | – | – | 7.8±5.5 | 8.5±6.2 | – | – |
| EROA, cm2 | 0.31±0.10 | 0.31±0.11 | 0.41±0.15 | 0.40±0.15 | 0.23 (0.20-0.30) | 0.23 (0.19-0.29) |
| RV, mL | 45±13 | 45±14 | – | – | 35.4 (28.9-43.9) | 35.6 (28.2-42.5) |
| LVEDV, mL | 255.6±63 | 258.8±71 | 194.4±69.2 | 191.0±72.9 | 200 (153-249) | 206 (158-250) |
| LVEF, % | 33.3±6.5 | 32.9±6.5 | 31.3±9.1 | 31.3±9.6 | 32 (27-36) | 31 (25-37) |
| Loop diuretics | 151 (99.3) | 149 (98.0) | 270 (89.4) | 277 (88.8) | 239 (95.6) | 243 (95.3) |
| ACEi/ARB | 111 (73.0) | 113 (74.3) | 204 (67.8) | 187 (60.0) | 190 (76.0) | 186 (72.9) |
| ARNi | 14 (10.0) | 17 (12.1) | 13 (4.3) | 9 (2.9) | 40 (16.0) | 28 (11.0) |
| MRA | 86 (56.6) | 80 (53.0) | 153 (50.7) | 155 (49.7) | 200 (80.0) | 215 (84.3) |
| Beta blockers | 134 (88.2) | 138 (90.8) | 275 (91.1) | 280 (89.7) | 238 (95.2) | 246 (96.5) |
| SGLT2i | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 24 (9.6) | 22 (8.6) |
| CRT | 46 (30.5) | 35 (23.0) | 115 (38.1) | 109 (34.9) | 77 (30.9) | 68 (26.7) |
| Continuous variables are summarised as mean±standard deviation or median (interquartile range), as appropriate. Categorical variables are summarised as counts (proportions). ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin II receptor blocker; ARNi: angiotensin receptor-neprilysin inhibitor; COAPT: Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation; CRT: cardiac resynchronisation therapy; eGFR: estimated glomerular filtration rate; EROA: effective regurgitant orifice area; EuroSCORE: European System for Cardiac Operative Risk Evaluation; GDMT: guideline-directed medical therapy; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; MITRA-FR: Percutaneous Repair with the MitraClip Device for Severe Secondary Mitral Regurgitation; MRA: mineralocorticoid receptor antagonist; NT-proBNP: N-terminal pro B-type natriuretic peptide; NYHA: New York Heart Association; RESHAPE-HF2: Randomised Study of the MitraClip Device in Heart Failure Patients with Clinically Significant Functional Mitral Regurgitation 2; RV: regurgitant volume; SGLT2i: sodium-glucose co-transporter-2 inhibitor; STS: Society of Thoracic Surgeons; TEER: transcatheter edge-to-edge repair | ||||||
Primary outcome
At the 24-month follow-up, TEER plus GDMT was associated with a significant reduction in death or hospitalisation due to heart failure compared with GDMT alone by one-stage analysis (HR 0.72, 95% CI: 0.56-0.92; p=0.010) based on reconstructed time-to-first event individual patient data (Figure 1). The reconstructed 2-year rate of survival free from events was 51.3% in the TEER plus GDMT group and 38.1% in the GDMT-alone group (Figure 1). However, the two-stage random-effects analysis without and with 95% CI correction showed a non-significant difference between groups (random-effects: HR 0.72, 95% CI: 0.51-1.00; p=0.052; random-effects with 95% CI correction: HR 0.72, 95% CI: 0.34-1.50; p=0.192) (Figure 1). While the relative weight of each trial was balanced, between-trial heterogeneity was substantial (I2=80.3%; p=0.006), mainly due to the substantial differences in the effects of the COAPT (HR 0.57, 95% CI: 0.45-0.71; p<0.001) and RESHAPE-HF2 trials (HR 0.65, 95% CI: 0.50-0.85; p<0.001), supporting a benefit of TEER plus GDMT over GDMT alone, and the effect of the MITRA-FR trial (HR 1.01, 95% CI: 0.77-1.34; p=0.944), indicating no significant difference between the treatment groups (Figure 1). The high heterogeneity resulted in a prediction interval crossing the null, highlighting the uncertainty in the effect size of a new trial according to the available information (Figure 1). These findings were outlined by the leave-one-out analysis, in which heterogeneity was no longer detectable after removal of the MITRA-FR trial (Supplementary Figure 3). The exclusion of either the COAPT trial or the RESHAPE-HF2 trial produced largely non-significant differences between treatment groups (Supplementary Figure 3).
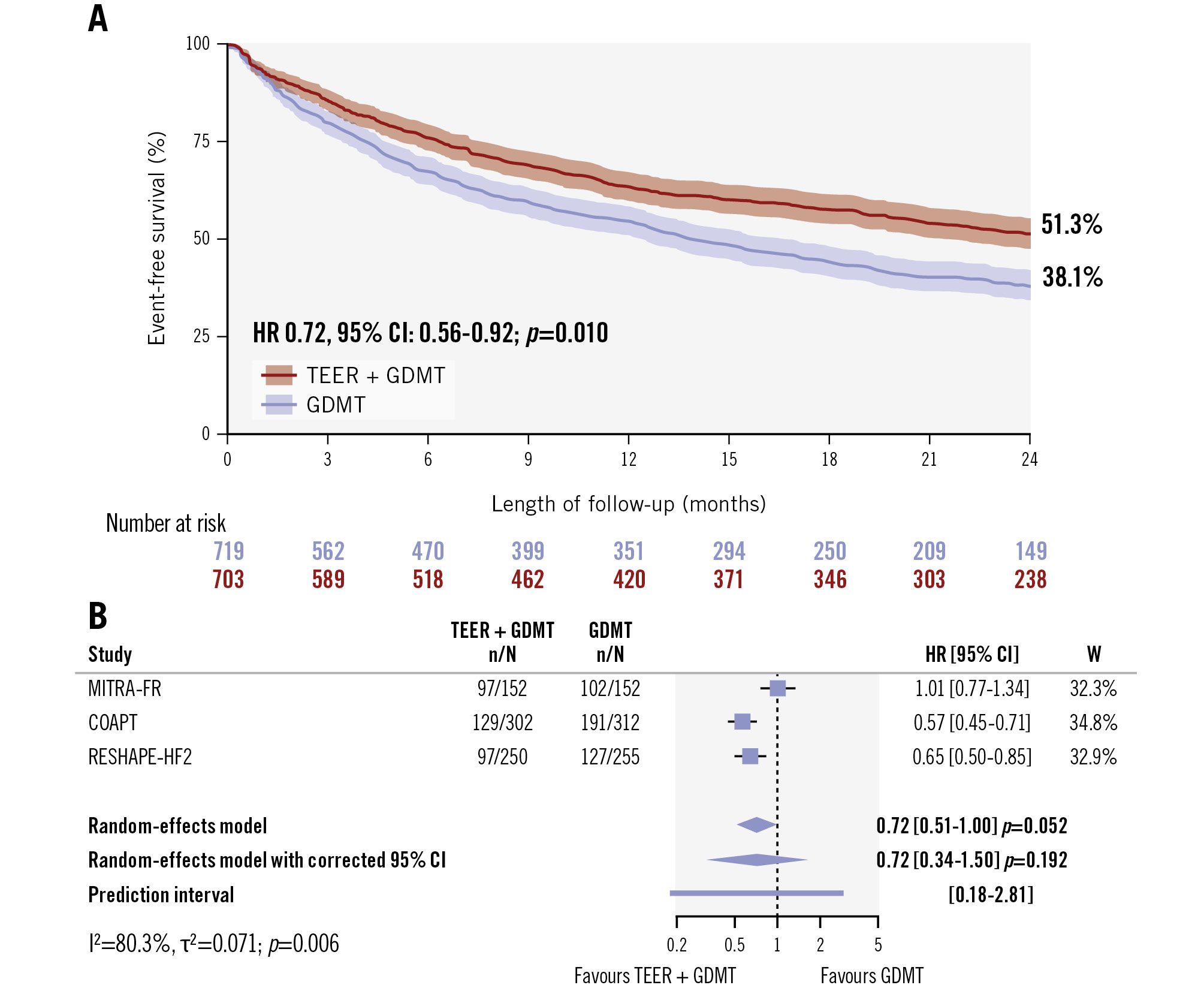
Figure 1. All-cause death or hospitalisation due to heart failure between TEER plus GDMT and GDMT alone. A) Event-free survival by the Kaplan-Meier method after combination of reconstructed time-to-event individual patient data and the one-stage meta-analysis results. B) Two-stage meta-analysis results without and with 95% confidence interval correction by the Hartung-Knapp method and the prediction interval. CI: confidence interval; GDMT: guideline-directed medical therapy; HR: hazard ratio; TEER: transcatheter edge-to-edge repair; W: weight
Secondary outcomes
The key secondary outcome of first hospitalisation for heart failure at 24 months was significantly reduced in patients assigned to TEER plus GDMT compared with those assigned to GDMT alone by one-stage analysis (HR 0.65, 95% CI: 0.48-0.88; p=0.006) based on reconstructed time-to-first event individual patient data (Figure 2). The reconstructed 2-year survival free from hospitalisation due to heart failure was 61.4% in the TEER plus GDMT group and 47.2% in the GDMT-alone group (Figure 2). The two-stage analysis showed consistent results (HR 0.66, 95% CI: 0.45-0.96; p=0.031) (Figure 2). However, after conservative correction of the 95% CI by the Hartung-Knapp method, the difference between treatments was no longer significant (HR 0.66, 95% CI: 0.29-1.52; p=0.164) as a result of the substantial between-trial heterogeneity (I2=81.2%; p=0.005) (Figure 2). The 95% prediction interval crossed the null, highlighting the uncertainty in the predicted effect size of a new trial according to the available information (Figure 2). The leave-one-out analysis confirmed that the exclusion of either the COAPT trial or the RESHAPE-HF2 trial led to non-significant differences between treatment groups, and the exclusion of the MITRA-FR trial rendered heterogeneity no longer detectable (Supplementary Figure 3).
Death was not significantly different between treatment groups (HR 0.76, 95% CI: 0.57-1.01; p=0.056), though there was a numerical trend toward a mortality reduction in patients assigned to TEER plus GDMT compared with those assigned to GDMT alone (Figure 3). Between-trial heterogeneity was moderate (I2=52.0%; p=0.124) and, after excluding MITRA-FR, there was a significant reduction in death associated with TEER plus GDMT compared with GDMT alone (HR 0.66, 95% CI: 0.53-0.83; p<0.001) (Supplementary Figure 3). Cardiovascular death was not significantly different between patients assigned to TEER plus GDMT compared with those assigned to GDMT alone (HR 0.77, 95% CI: 0.56-1.06; p=0.110), though a numerical trend was observed towards a cardiovascular mortality reduction associated with TEER plus GDMT compared with GDMT alone (Figure 3). Between-trial heterogeneity was moderate (I2=53.5%; p=0.117), and after excluding MITRA-FR, a significant reduction was observed associated to cardiovascular death with TEER plus GDMT compared with GDMT alone (HR 0.68, 95% CI: 0.49-0.96; p=0.029) (Supplementary Figure 3).
Accounting for both first and recurrent hospitalisations due to heart failure, the results remained consistent with the time-to-first event analysis, using the standard analysis (HR 0.62, 95% CI: 0.48-0.80; p<0.001). However, after correcting the 95% CI, the difference was no longer statistically significant (HR 0.62, 95% CI: 0.34-1.13; p=0.075) (Figure 3). In line with the other outcomes, between-trial heterogeneity was essentially attributable to MITRA-FR, and both the COAPT and RESHAPE-HF2 trials were required to reach a significant difference between treatment groups (Supplementary Figure 3). Considering the maximum available follow-up of the COAPT trial (5 years), the results marginally failed to remain statistically significant (HR 0.70, 95% CI: 0.48-1.01; p=0.057) (Figure 3).
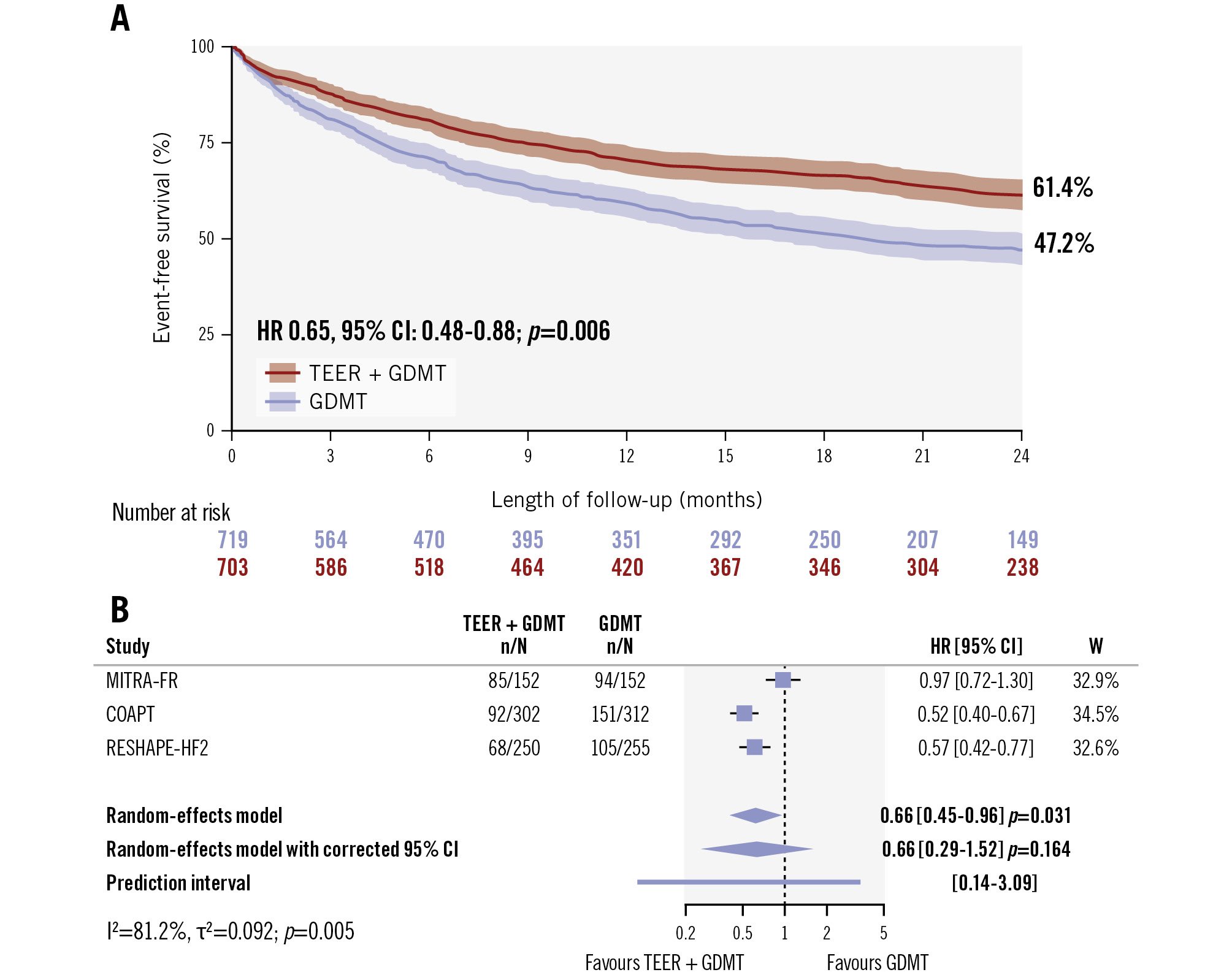
Figure 2. Hospitalisation due to heart failure between TEER plus GDMT and GDMT alone. A) Event-free survival by the Kaplan-Meier method after combination of reconstructed time-to-event individual patient data and the one-stage meta-analysis results. B) Two-stage meta-analysis results without and with 95% confidence interval correction by the Hartung-Knapp method and the prediction interval. CI: confidence interval; GDMT: guideline-directed medical therapy; HR: hazard ratio; TEER: transcatheter edge-to-edge repair; W: weight
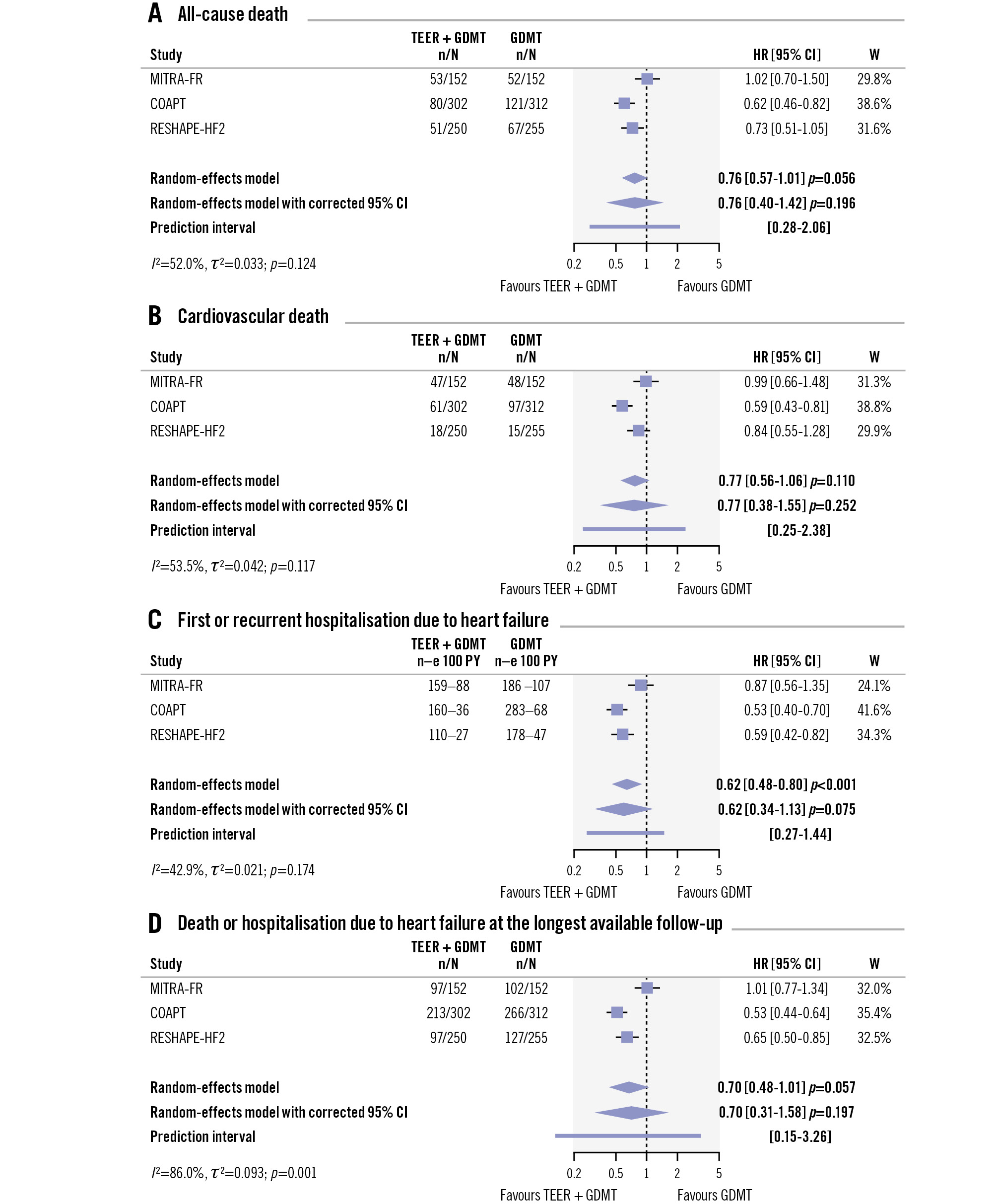
Figure 3. All-cause death and cardiovascular death between TEER plus GDMT and GDMT alone. CI: confidence interval; GDMT: guideline-directed medical therapy; HR: hazard ratio; TEER: transcatheter edge-to-edge repair; W: weight
Discussion
This meta-analysis integrates data from the COAPT, MITRA-FR, and RESHAPE-HF2 trials, offering a comprehensive and up-to-date evaluation of mitral valve TEER performed in patients with symptomatic moderate-to-severe FMR61112131415. By pooling the entire evidence from randomised clinical trials accrued thus far, the reconstructed time-to-event individual patient data one-stage analysis demonstrated that TEER plus GDMT is more effective than GDMT alone in reducing a composite of death or first hospitalisation due to heart failure as well as the individual outcome of first hospitalisation due to heart failure (Central illustration). However, while the two-stage analysis for first hospitalisation due to heart failure was consistent with the one-stage analysis, the two-stage analysis for death or first hospitalisation due to heart failure was not associated with a significant difference between treatment groups. In addition, substantial between-trial heterogeneity between treatment groups was observed, and consequently, the conservatively corrected 95% CIs and the prediction intervals denoted significant residual uncertainty, highlighting the need for more data7.
MITRA-FR was the first randomised trial comparing TEER plus GDMT with GDMT alone for the treatment of FMR11. In this trial, the primary composite endpoint of 1-year all-cause death or hospitalisation for heart failure and the individual endpoints of all-cause death and hospitalisation due to heart failure did not support a prognostic improvement after invasive treatment, though TEER was not associated with safety concerns11. The contrasting conclusions of COAPT renewed enthusiasm for TEER as an intervention to improve the prognosis of patients with symptomatic moderate-to-severe FMR who are deemed unsuitable for surgery, likely saving this therapeutic option from oblivion12. In the COAPT trial, the primary endpoint of hospitalisation due to heart failure at 2-year follow-up was significantly lower in patients assigned to TEER plus GDMT than in those assigned to GDMT alone, with annualised incidences of 35.8% per patient-years and 67.9% per patient-years, respectively12.
Reconciling the conclusions between the MITRA-FR and COAPT trials is challenging. Beyond some observed differences in baseline characteristics and comorbidity burden, the selection of patients to be included in the trial and the management of heart failure before and after TEER may have played a role in the dissimilar conclusions of the two trials. In the COAPT trial, FMR severity was assessed by a core laboratory, and an independent multidisciplinary committee, including heart failure specialists, verified the eligibility of inclusion of each patient based on whether heart failure treatments at the maximum tolerated dose were employed without tangible clinical improvements and excluded a reduction in mitral regurgitation severity during the intensified run-in phase. In the MITRA-FR trial, less standardised procedures may have led to a more liberal selection of patients. Against this background, some differences in the medications used before TEER between MITRA-FR and COAPT may indicate higher heterogeneity in the stage of FMR disease, though they cannot be directly and definitively linked with diverging outcomes. In this context, a different proportion of patients on cardiac resynchronisation therapy may also have had an influence. Regarding patient selection, it is fundamental to recognise that, on equal terms of baseline left ventricular ejection fraction and ischaemic aetiology, some echocardiographic inclusion criteria in MITRA-FR led to a less restrictive inclusion of patients compared with COAPT. Specifically, patients enrolled in the MITRA-FR trial showed larger left ventricular end-diastolic volumes and lower effective regurgitant orifice areas compared with those enrolled in the COAPT trial. These findings provided the groundwork for the hypothesis that patients enrolled in the MITRA-FR trial more frequently had proportionate FMR, while those enrolled in the COAPT trial more frequently had disproportionate FMR16. Specifically, in some patients with FMR, the effective orifice area is proportionate to the degree of left ventricular dilatation, allowing for a more effective response to medications that reduce left ventricular end-diastolic volume16. In contrast, in some patients with FMR, the effective orifice area is disproportionately higher than the degree of left ventricular end-diastolic volume, implying a narrower margin for treatment with medications and higher benefits of interventions on the valve16. While useful to reconcile COAPT and MITRA-FR, this framework has not been robustly validated in retrospective TEER cohorts and should be regarded as hypothesis-generating1718.
These differences likely supported selection at a different stage in the natural history of FMR, and long-term follow-up results were consistent with these considerations1920. Although procedural efficacy in the two trials was high, exceeding 90%, regardless of the treatment by TEER plus GDMT or GDMT alone, patients in the MITRA-FR trial at 2-year follow-up exhibited a lower rate of NYHA Class ≤II, larger left ventricular end-diastolic volumes, and less durable FMR grade ≤2 reduction compared with those in the COAPT trial16. The variability of GDMT monitoring and adherence after TEER further complicates the interpretation of outcomes. In COAPT, more stringent GDMT assessment protocols were mandated, including functional and laboratory examinations12. In MITRA-FR, less rigorous monitoring raises questions about the extent of GDMT optimisation and possible therapeutic differences between treatment groups11.
The recent publication of the early terminated RESHAPE-HF2 trial results has mitigated the uncertainty surrounding TEER efficacy. In this trial, 24-month cardiovascular death or hospitalisation due to heart failure was significantly lower in patients assigned to TEER plus GDMT than in those assigned to GDMT alone, with annualised incidences of 37.0% per patient-years and 58.9% per patient-years, respectively6. Despite significant challenges, including an accrual lasting approximately 8 years, the predominant recruitment from two countries (Greece and Poland), accounting for nearly 80% of patients, and changes in GDMT for heart failure during the trial, RESHAPE demonstrated consistent long-term clinical benefits of TEER across outcomes. Specifically, first-time and recurrent event analyses of hospitalisation due to heart failure and the mean change in Kansas City Cardiomyopathy Questionnaire - Overall Summary score supported the significant improvements associated with TEER. These results were consistent with the sustained improvements in exercise capacity and quality of life previously observed in the COAPT trial12.
While the COAPT trial showed a significant reduction in all-cause death, the RESHAPE-HF2 trial did not confirm this conclusion612. In the present meta-analysis, the lack of significant differences between TEER plus GDMT and GDMT alone in terms of all-cause death and cardiovascular death may indicate that TEER benefits primarily involve symptoms and quality of life. Nevertheless, it should also be acknowledged that numerical trends towards significant reductions in all-cause and, to a lesser extent, cardiovascular death may be a function of insufficient statistical power for these individual outcomes. These findings underscore the need for more data to provide definitive conclusions6.
Recently, another meta-analysis of randomised trials explored the comparison of TEER plus GDMT versus GDMT alone in patients with significant FMR7. In the present study, we used different methods compared with the meta-analysis by Anker and colleagues. In particular, unlike the previous meta-analysis, we used one-stage analyses based on reconstructed time-to-event individual patient data and frailty models. In addition, slight differences in two-stage random-effects meta-analyses may be attributed to different parametrisation7. In our study, the 95% CIs of summary estimates computed by frailty models were narrower than those of two-stage analyses – and therefore than those of standard random-effects analyses in the meta-analysis by Anker and colleagues – because the one-stage analysis generally is more convenient in terms of statistical power. However, beyond these statistical differences, our findings are broadly consistent with the meta-analysis by Anker and colleagues, and the main distinction lies in the critical interpretation of the substantial between-trial heterogeneity7. Notably, even in the meta-analysis by Anker and colleagues, none of the summary estimates obtained using random-effects models with conservative 95% CI adjustment via the Hartung-Knapp method reached statistical significance. Despite this, the authors concluded that the results were sufficiently consistent and presented adjusted 95% CIs as merely “broader”, even though they were no longer supportive of a significant difference7.
Finally, the MATTERHORN trial has recently demonstrated not only the non-inferiority of TEER compared with surgical mitral valve repair or replacement in high-risk patients but also its superiority in terms of major procedural complications and hospital length of stay21. Notably, in this trial, symptom relief after TEER remained stable at 1-year follow-up, with no significant differences compared with surgical mitral valve repair or replacement21. These findings have been endorsed by contemporary guidelines, which recommend TEER to reduce heart failure hospitalisations and improve quality of life in symptomatic ventricular FMR receiving optimised GDMT (Class I, Level of Evidence A)22. Longer-term data from MATTERHORN and similar trials will be crucial for assessing the durability of TEER compared with surgery, thereby consolidating its role as a sustainable alternative to surgery in high-risk patients who meet guideline indications212324.
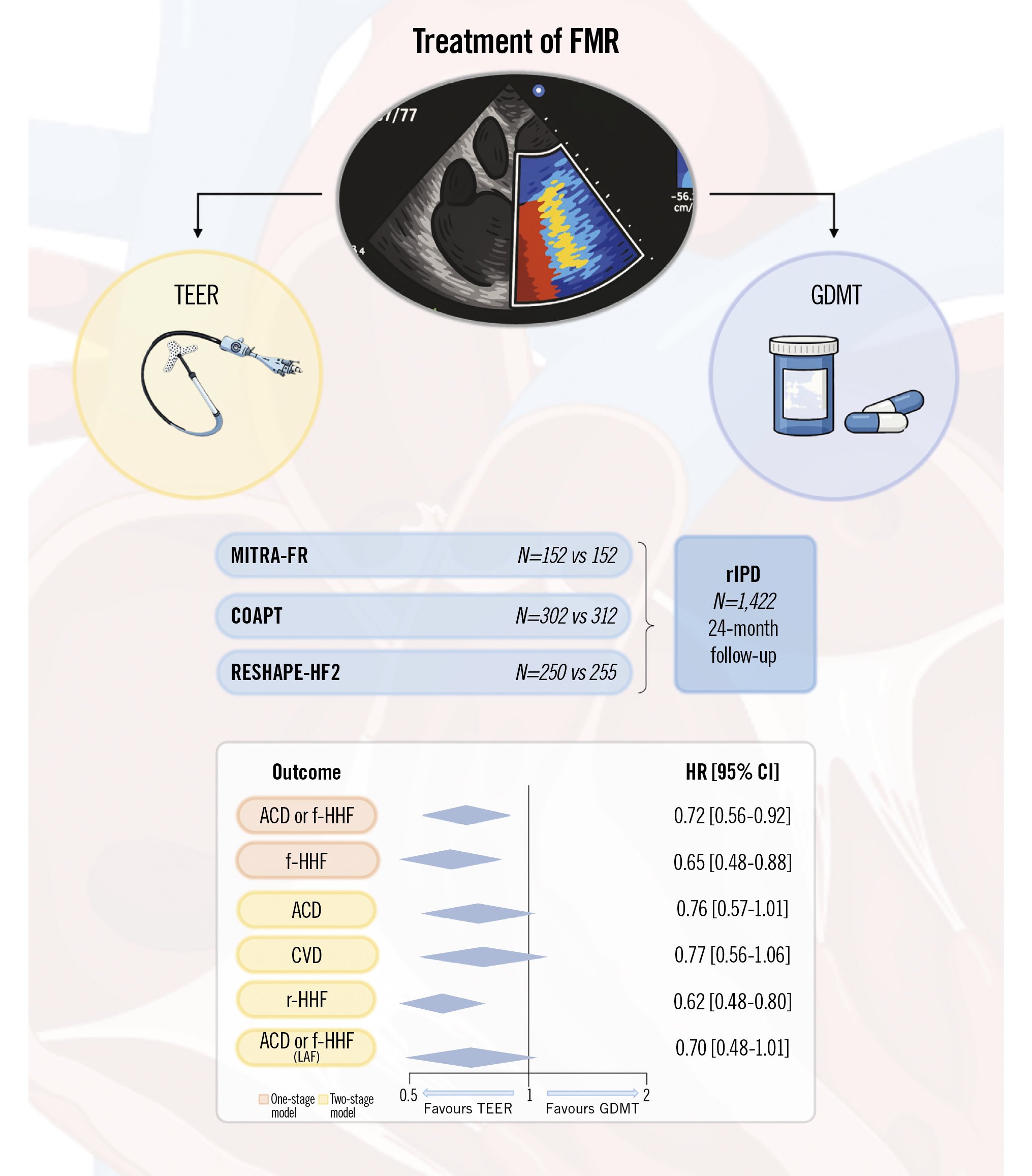
Central illustration. Randomised trials and pooled effects of TEER plus GDMT versus GDMT alone for moderate-to-severe FMR. ACD: all-cause death; CI: confidence interval; CVD: cardiovascular death; f-HHF: first hospitalisation due to heart failure; FMR: functional mitral regurgitation; GDMT: guideline-directed medical therapy; HR: hazard ratio; LAF: longest available follow-up; r-HHF: first or recurrent hospitalisation for heart failure; rIPD: reconstructed individual patient data; TEER: transcatheter edge-to-edge repair
Limitations
The results of this meta-analysis should be interpreted considering the following limitations. First, this meta-analysis was based on study-level and reconstructed time-to-event data, implying limited flexibility and dependency on original reporting. Nevertheless, the randomised design ensured a negligible influence of individual patient associations on the outcomes between groups, and all trials showed overall high methodological quality and an acceptable extent of reported data. Second, the composite primary endpoint of RESHAPE-HF2 included cardiovascular death instead of all-cause death6 for the one-stage analysis. Third, between-trial heterogeneity was high, likely implying some differences in the selection of patients. Access to individual patient data may provide more insights into this assumption and possible causal associations. Fourth, GDMT may have varied across trials, changes in the guidelines during the prolonged course of trials may have influenced GDMT, and more recently approved medications for heart failure were available only to the last patients enrolled in the RESHAPE-HF2 trial6. Fifth, the long recruitment periods across trials likely reveal accurate screening and selection of patients, posing some considerations about the generalisability of results. Finally, only one TEER system was employed across trials, and the results may not apply to other devices. Additionally, although new transcatheter therapies for FMR, including annuloplasty and valve replacement techniques, may offer alternative options to or complement TEER, strategies involving a combination of interventions or a bioprosthetic valve still warrant randomised trials18.
Conclusions
In patients with FMR, TEER plus GDMT is associated with a significant reduction in death or hospitalisation due to heart failure and hospitalisation due to heart failure compared with GDMT alone. However, the observation of high between-trial heterogeneity translated into non-significant between-group differences in these outcomes when employing more conservative methods. Although a clear numerical trend favouring TEER plus GDMT was observed, all-cause death and cardiovascular death did not significantly differ between treatment groups. The exclusion of MITRA-FR was instrumental in eliciting improved survival in the TEER plus GDMT group compared with the GDMT-alone group. Whether these findings reflect differences in patient selection warrants clarification by further research.
Impact on daily practice
In patients with symptomatic functional mitral regurgitation on optimal guideline-directed medical therapy, transcatheter edge-to-edge repair (TEER) reduces the long-term incidence of the composite endpoint of death or hospitalisation due to heart failure and the rate of heart failure-related hospitalisation. Despite the substantial heterogeneity driven by the MITRA-FR trial, which emphasises the need for patient selection, the consistent risk reductions observed in COAPT and RESHAPE-HF2 confirm that TEER achieves superior long-term outcomes over guideline-directed medical therapy.
Conflict of interest statement
D. Capodanno declares speaker fees or honoraria from Daiichi Sankyo, Sanofi, and Terumo. The other authors have no relevant conflicts of interest to declare related to the topic of this manuscript. The Guest Editor reports consultancy fees from Novartis and Meril Life Sciences; and speaker honoraria from Meril Life Sciences.
Supplementary data
To read the full content of this article, please download the PDF.

