Cory:
Unlock Your AI Assistant Now!
Transcatheter tricuspid valve replacement (TTVR) is an alternative treatment modality for a subset of patients with severe tricuspid regurgitation who are unsuitable for traditional surgery or transcatheter edge-to-edge repair. One TTVR device, the EVOQUE valve (Edwards Lifesciences), has recently become the first TTVR device to receive U.S. Food and Drug Administration approval for the treatment of severe tricuspid regurgitation. Reported midterm outcomes of the EVOQUE valve have been favourable, demonstrating reductions in tricuspid regurgitation, symptom burden, and heart failure hospitalisation rates12. However, there remains a paucity of data on long-term outcomes following TTVR with the EVOQUE valve. A 69-year-old female patient with a background history of rheumatic heart disease, mitral valve commissurotomy, and subsequent mechanical mitral valve replacement (St. Jude Medical 25 mm mechanical prosthesis) presented with New York Heart Association (NYHA) IV dyspnoea, refractory peripheral oedema, ascites, and multiple recent heart failure hospitalisations. Transthoracic echocardiography (TTE) revealed a left ventricular ejection fraction of 50%, a dilated right ventricle (RV; basal diameter of 46 mm) with mildly reduced function (tricuspid annular plane systolic excursion [TAPSE] of 9 mm), a well-functioning mitral prosthesis with trivial valvular mitral regurgitation, severe tricuspid regurgitation, and pulmonary hypertension, with an RV systolic pressure of 48 mmHg (Moving image 1). She was pacing dependent, having had a dual-chamber permanent pacemaker implanted prior to undergoing atrioventricular node ablation. She was felt to have prohibitive surgical risk and therefore underwent TTVR with a 44 mm EVOQUE valve (Moving image 2). Postprocedural transoesophageal echocardiography (TOE) showed trivial tricuspid regurgitation and a tricuspid valve mean gradient of 2 mmHg (Figure 1). Her immediate postoperative course was uncomplicated, and at 30-day follow-up, the patient reported NYHA II dyspnoea. She was prescribed aspirin 81 mg once daily and warfarin for 12 months post-TTVR, followed by warfarin monotherapy for life. At 5-year follow-up, the patient reported NYHA II dyspnoea. Her six-minute walk test distance had increased from 115 metres preprocedure to 450 metres. The patient had had only one hospitalisation in the 5 years following EVOQUE implantation; this was due to multifocal pneumonia and sepsis. The TOE performed during that admission revealed a well-seated 44 mm EVOQUE valve with a mean gradient of 4 mmHg, and trivial tricuspid regurgitation with no evidence of paravalvular regurgitation (Moving image 3). Computed tomography (CT) showed no evidence of structural valve degeneration. TTE showed a normal RV size, with a basal RV diameter of 41 mm and mildly reduced RV function (TAPSE of 15 mm). Her baseline conduction remained unchanged, despite the RV lead having been jailed during the procedure. She remained pacing dependent and underwent a routine generator change 4 years after TTVR. In this report of the 5-year follow-up TOE and CT imaging of a patient undergoing TTVR with the EVOQUE device, there was normal leaflet function, with no evidence of degeneration. Residual tricuspid regurgitation was trivial to mild. The patient had had no heart failure hospitalisations and had noted a significant improvement in symptom burden. Further studies are needed to evaluate the long-term efficacy and safety of TTVR with the EVOQUE valve. 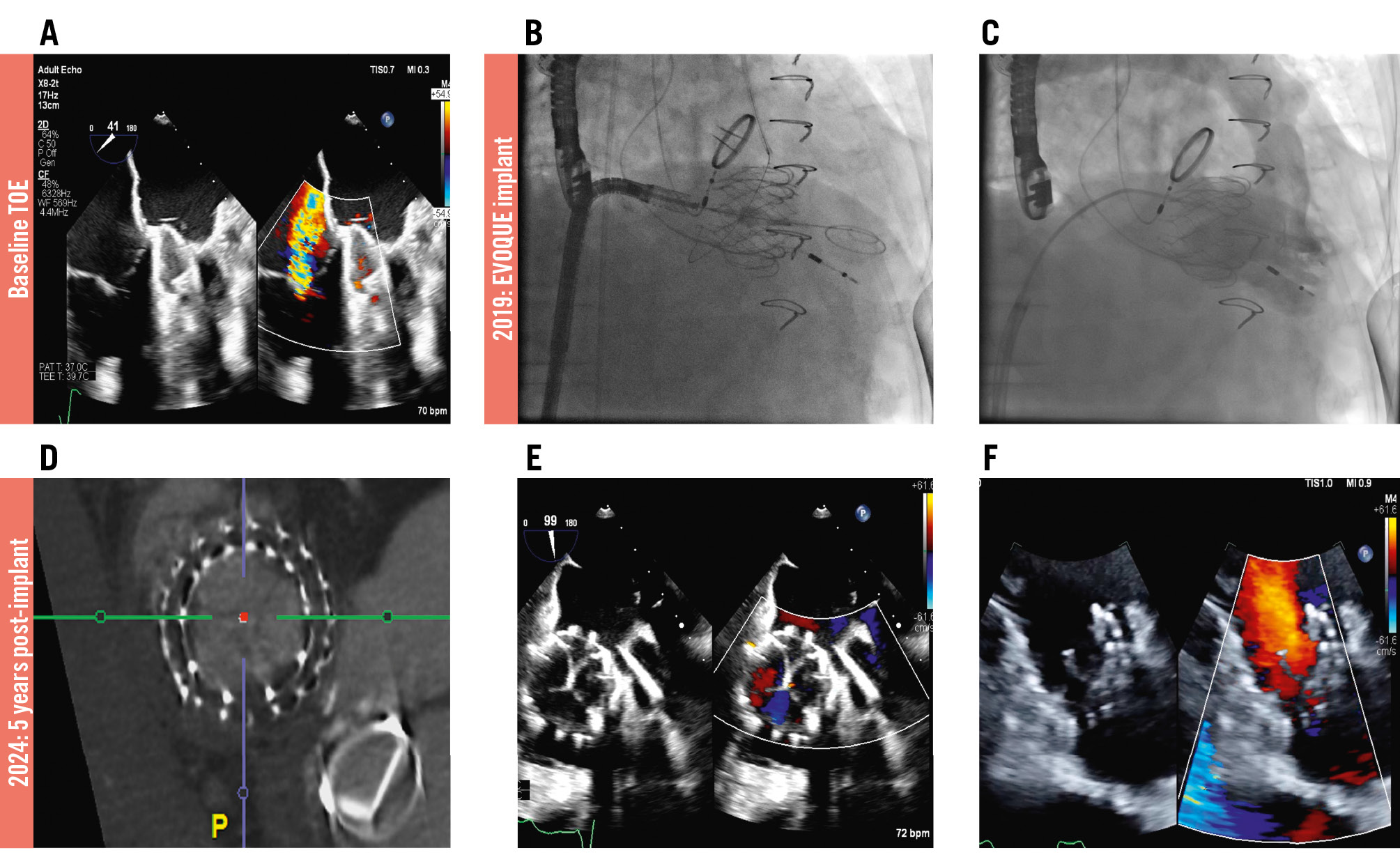
Figure 1. Five-year follow-up of transcatheter tricuspid valve replacement with the EVOQUE valve. A) Preprocedural transoesophageal echocardiogram shows severe tricuspid regurgitation. B) Fluoroscopy of the EVOQUE valve implant shows the deployed valve. C) Right ventricular angiogram via a pigtail catheter immediately post-implant shows minimal residual TR. D) CT 5 years post-implant shows no evidence of structural valve deterioration. E,F) Transoesophageal and transthoracic echocardiogram images 5 years post-TTVR shows minimal residual TR. CT: computed tomography; TR: tricuspid regurgitation; TTVR: transcatheter tricuspid valve replacement
Conflict of interest statement
J.G. Webb is a consultant for Edwards Lifesciences; and receives research funding from Edwards Lifesciences, Medtronic, and Boston Scientific. The other authors have no relevant conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Preprocedural transthoracic echocardiogram showing severe tricuspid regurgitation.
Moving image 2. Fluoroscopic images from the implant procedure, showing the initial RV angiogram with severe tricuspid regurgitation, followed by deployment of the EVOQUE valve over a SAFARI² XS wire in the right ventricle. Initial deployment of the nine ventricular anchors followed by final deployment of the EVOQUE valve and the final RV angiogram following implant showing a well-placed EVOQUE valve with minimal residual tricuspid regurgitation.
Moving image 3. Transoesophageal echocardiogram at 5 years post-implant showing the well-seated EVOQUE valve with trivial tricuspid regurgitation and no paravalvular regurgitation.

